��Ŀ����
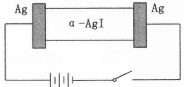
�о����֣�������ʯī������������������������CaF2��CaO������ʣ�����ͼʾװ�û�ý����ƣ����Ը�Ϊ��ԭ����ԭ���������Ʊ������ѡ�����˵������ȷ����
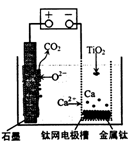

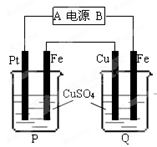
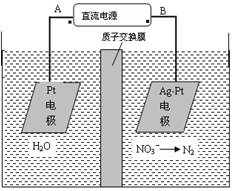
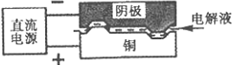
| A��������CaF2-CaO����Ca(NO3)2��ҺҲ���Դﵽ��ͬĿ�� |
B�������ĵ缫��ӦʽΪ��C+2O2��-4e�� CO2�� CO2�� |
| C�����Ʊ�������ǰ������װ����CaO���������� |
| D������Ǧ��������װ�õĹ����Դ����+"������Ӧ����Pb�缫 |
B
��������������ƺܻ��ã����ˮ��Ӧ����˲�������Һ������ʣ�Aѡ�������ʯī������������������������CaF2-CaO������ʣ���������������Ӧ�����������ƽ���������ԭ��Ӧ������ͼʾ����ɿ�֪���������ɶ�����̼���壬�ǵ�����е�������ʧ������������������������ʯī��Ӧ���ɵĶ�����̼�����Ե缫��ӦΪ��2O2��-4e��=O2����C+2O2��-4e��=CO2����Bѡ����ȷ���Ʊ�TiO2ʱ���ڵ��۷������·�Ӧ��2CaO�T2Ca+O2����2Ca+TiO2
 Ti+2CaO���ɴ˿ɼ���CaO�������䣬Cѡ���������Ǧ��������װ�õĹ����Դ����+"������Ӧ����PbO2�缫��Dѡ�����
Ti+2CaO���ɴ˿ɼ���CaO�������䣬Cѡ���������Ǧ��������װ�õĹ����Դ����+"������Ӧ����PbO2�缫��Dѡ�����
��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ
 CaO�������,������ͼ��ʾװ�û�ý�����,���Ը�Ϊ��ԭ����ԭ���������Ʊ������ѡ�����˵������ȷ����(����)
CaO�������,������ͼ��ʾװ�û�ý�����,���Ը�Ϊ��ԭ����ԭ���������Ʊ������ѡ�����˵������ȷ����(����)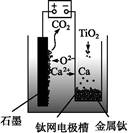
 CO2��
CO2��