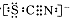��Ŀ����
�����仯���������������о�����Ҫ�����á�
��1������Ԫ�����ڱ��е�λ���� ��
��2����֪�縺�Ե���ֵ��ʾԭ�ӶԵ���������������Դ�С�������Ǽ���ԭ�ӵĵ縺����ֵ��
| Ԫ�� | �� | þ | �� | �� |
| �縺�� | 0.9 | 1.2 | �� | 1.8 |
�����ĵ縺�Ԧֵķ�Χ�� ��
�ڵ縺�Ե���ֵ��Ԫ�ؽ����ԵĹ�ϵ�� ��
������ʵ���ܱȽ�þ�����Ľ�����ǿ������ ��
a���ⶨþ�����ĵ�����ǿ��
b���ⶨ�����ʵ���Ũ�ȵ�Al2(SO4)3��MgSO4��Һ��pH
c����0.1 mol/LAlCl3��0.1 mol/L MgCl2�мӹ���NaOH��Һ
��3�����ȷ��dz��õĽ���ұ������֮һ��
��֪��4Al(s)��3O2(g)��2Al2O3(s) ��H1��-3352 kJ/mol
Mn(s)��O2(g)��MnO2(s) ��H2�� -521 kJ/mol
Al��MnO2��Ӧұ������Mn���Ȼ�ѧ����ʽ�� ��
��4��ұ��������ʱ����ʯī���缫�������Al2O3��Һ̬���ڣ�������������� ���õ����������У�����ʯī��Ҫ���ϲ��䣬��ϵ缫��Ӧ˵����ԭ���� ��
��14�֣�ÿ��2�֣�
��1����3���ڵڢ�A��
��2���ٵ縺����ֵԽ��Ԫ�صĽ�����Խ��
�� 1.2���֣�1.8
�� c
��3��4Al(s)�� 3MnO2(s)��3Mn(s)��2Al2O3(s) ��H���C1789 kJ/mol
��4����
������������Ӧ2O2-�C 4e-��O2����C��O2��CO2��ʯī���ڱ�������Ҫ���ϲ���
���������������1��AlΪ13��Ԫ�أ�λ�ڵ�3���ڵڢ�A�塣
��2���ٸ��ݵ縺�Եı仯���ɣ����ĵ縺�Խ���Mg��Si֮�䡣
�ڵ縺����ֵԽ��ԭ�ӶԵ�����������Խ��ʧ����Խ�ѣ����Խ�����Խ����
��a����������縺���أ�����b�������ʵ���Ũ�ȵ�Al2(SO4)3��MgSO4��Һ��SO42?Ũ�Ȳ�ͬ������c���ӹ���NaOH��Һ��AlCl3��Һ�ȳ��ֳ������ܽ⣬˵��Al(OH)3�����ԣ�����������Mg����ȷ��
��3������д����ѧ����ʽ��ע��״̬��Ȼ����ݸ�˹�������ʱ䣺?H=?H1�C3?H2=" �C1789" kJ?mol?1������д���Ȼ�ѧ����ʽ��
��4��Al3+�������õ�������Al������O2?�õ�������O2���ڽϸ��¶�����ʯī��Ӧ����CO2��ʯī��ر����ģ�������Ҫ���ϲ��䡣
���㣺 ���⿼��Ԫ�����������������ڱ����Ȼ�ѧ����ʽ����д������ԭ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1����FeCl3��Һ�м���a g��ͭ�ۣ�����ʹ֮ȫ���ܽ⣬��Һ��һ���е���������_________�������е��������� ��������Ӧ�����ӷ���ʽΪ ���������м��� b g���ۣ���ַ�Ӧ����˵�����c g�����ܷ�����Ӧ�����ӷ���ʽΪ ������֪ a��b��c���� c��������_________��
��2������Na2SO3��Һ��ϡH2SO4���밴Ҫ������±���
| ���� | ���� | ������ |
| 1 | �üס�����֧�Թֱܷ�ȡ������Һ�������� | |
| 2 | �ý�ͷ�ι�����Թ�����εμ�BaCl2��Һ�������� | |
| 3 | �� | ������� ������ �� ������� ������ �� |
˵������3������ʹ�������κ�������������ͷ�ιܣ����Լ���
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã�
�ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | �� | �� | �� | | | �� | |
��1���ɢ١�������Ԫ����ɵ���Է�������Ϊ28���л���Ŀռ乹���� ��
����������ˮ�����ӳɷ�Ӧ�Ļ�ѧ����ʽ�� ��
��2���õ���ʽ��ʾ�ܵļ��⻯����γɹ������£� ��
��3�� �����ʵ��Ƚ�Ԫ�آ��������Ե����ǿ���� _��
��4�� �â�Ԫ�صĵ������Ԫ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ������Ķ��Ե缫���ҽ���KOH��Һ�У��ڼ�ͨ��ٵĵ��ʣ��Ҽ�ͨ��ܵĵ��ʣ�����ĵ缫��ӦʽΪ�� ��
��5���ɱ��Т١��ۡ��ܡ��ޡ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
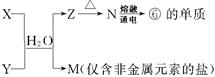
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ����ҵ�ϳ��õ��ʢ�ұ�����۵Ľ�����д���������͵��ʢ��ڸ����·�Ӧ�Ļ�ѧ����ʽ ��
 4AlCl3+3O2 ������ش��������⣺
4AlCl3+3O2 ������ش��������⣺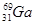 ����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��
����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��