��Ŀ����
�״���һ����Ҫ�Ļ���ԭ�ϣ�����һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ����
(1)��֪��CH3OH(g)=HCHO(g)+H2(g)�� ��H=+84 kJ��mol-1
2H2(g)+O2(g) ===2H2O(g)�� ��H=-484 kJ��mol-1
��ҵ�ϳ��Լ״�Ϊԭ����ȡ��ȩ����д��CH3OH(g)��O2(g)��Ӧ����HCHO(g)��H2O(g)���Ȼ�ѧ����ʽ��___��
(2)��ҵ�Ͽ������·����ϳɼ״�����ѧ����ʽΪCO(g)+2H2(g)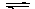 CH3OH(g)����֪ijЩ��ѧ���ļ����������±���
CH3OH(g)����֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | O��H |
����/ kJ��mol-1 | 348 | 413 | 436 | 358 | x | 463 |
��ش���������
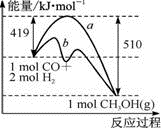
�ٸ÷�Ӧ�Ħ�S____(�>����<��)0����ͼ������a������b�Ĵ�ʩ��_______��
����֪CO�е�C��O֮��Ϊ�����������Ϊx kJ��mol-1����x=____��
(3)�ɼ״���������NaOH��Һ���ɵ������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε硣�ٸõ�ظ����ĵ缫��ӦʽΪ__��
�����Ըõ��Ϊ��Դ����ʯī���缫���200 mL�����������ӵ���Һ��
���� | Cu2+ | H+ | Cl? | SO42 �� |
c/mol��L-1 | 0.5 | 2 | 2 | 0.5 |
���һ��ʱ��������ռ�����ͬ���(��ͬ������)������ʱ(������Һ����ı仯���缫������ܴ��ڵ��ܽ�����)�������ռ�������������Ϊ____��

(4)���ˮ������CO2�����ϳ���(H2+CO)���ϸ��¶���(700~1 000 ��)����SOEC����缫��ʩ��һ����ֱ����ѹ��H2O��CO2����缫������ԭ��Ӧ����O2?��O2?�������ܵĹ������������ʲ㵽�����缫�������缫����������Ӧ�õ���O2������ͼ��֪AΪֱ����Դ��____(�������������)����д����H2OΪԭ������H2�ĵ缫��Ӧʽ��_______��


 �������AAPP��������Ⱦ�ϡ��л������м��塣��ϳ�·�����£�
�������AAPP��������Ⱦ�ϡ��л������м��塣��ϳ�·�����£�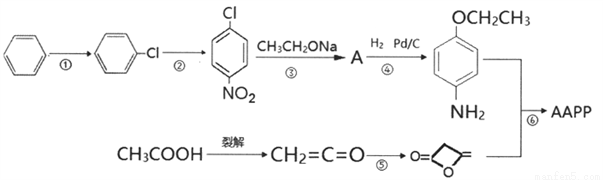
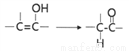
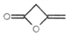 �ж���ͬ���칹�壬д�����ַ�������Ҫ���ͬ���칹��_________
�ж���ͬ���칹�壬д�����ַ�������Ҫ���ͬ���칹��_________