��Ŀ����
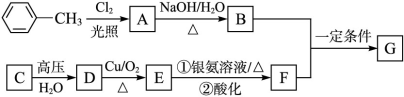
��֪�л���A��B��C��D��E��F��G������ת����ϵ������C�IJ�������������һ�����ҵ�ʯ�ͻ�����չˮƽ��G�ķ���ʽΪC9H10O2���Իش������й����⡣
��1��G�Ľṹ��ʽΪ__________��
��2��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Aת��ΪB��______��Cת��ΪD��__________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
D����E�Ļ�ѧ����ʽ��________________________��
B��F����G�Ļ�ѧ����ʽ��____________________��
��4����������������G��ͬ���칹����Ŀ��____�֣��ٱ�������3��ȡ��������������ȡ������ͬ�����ܹ������Ƶ�������Һ��Ӧ����������������������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ__________��____________��

��1��G�Ľṹ��ʽΪ__________��
��2��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Aת��ΪB��______��Cת��ΪD��__________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
D����E�Ļ�ѧ����ʽ��________________________��
B��F����G�Ļ�ѧ����ʽ��____________________��
��4����������������G��ͬ���칹����Ŀ��____�֣��ٱ�������3��ȡ��������������ȡ������ͬ�����ܹ������Ƶ�������Һ��Ӧ����������������������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ__________��____________��
��1�� ��2��ȡ����Ӧ���ӳɷ�Ӧ
��2��ȡ����Ӧ���ӳɷ�Ӧ
��3��2CH3CH2OH��O2 2CH3CHO��2H2O��CH3COOH��HOCH2
2CH3CHO��2H2O��CH3COOH��HOCH2
 CH3COOCH2
CH3COOCH2 ��H2O
��H2O

 ��2��ȡ����Ӧ���ӳɷ�Ӧ
��2��ȡ����Ӧ���ӳɷ�Ӧ��3��2CH3CH2OH��O2
 2CH3CHO��2H2O��CH3COOH��HOCH2
2CH3CHO��2H2O��CH3COOH��HOCH2
 CH3COOCH2
CH3COOCH2 ��H2O
��H2O
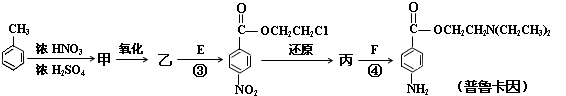
���������C�IJ�������������һ�����ҵ�ʯ�ͻ�����չˮƽ��˵��C����ϩ������ת����ϵ֪D���Ҵ���E����ȩ��F�����ᡣA��
 ,B�ǣ�
,B�ǣ� BF������������Ӧ�����G�ķ���ʽΪC9H10O2���Ƶ�GΪ
BF������������Ӧ�����G�ķ���ʽΪC9H10O2���Ƶ�GΪ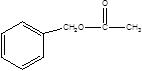 ������𰸷������£���1��G�Ľṹ��ʽΪ
������𰸷������£���1��G�Ľṹ��ʽΪ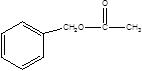 ����2��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Aת��ΪB��ȡ����Ӧ��Cת��ΪD���ӳɷ�Ӧ����3��D����E�Ļ�ѧ����ʽ��2CH3CH2OH��O2
����2��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Aת��ΪB��ȡ����Ӧ��Cת��ΪD���ӳɷ�Ӧ����3��D����E�Ļ�ѧ����ʽ��2CH3CH2OH��O2 2CH3CHO��2H2O��CH3COOH��H2O��B��F����G�Ļ�ѧ����ʽ��HOCH2
2CH3CHO��2H2O��CH3COOH��H2O��B��F����G�Ļ�ѧ����ʽ��HOCH2
 CH3COOCH2
CH3COOCH2 ��H2O����4����������һ������DZ����������������Ǽ���ij��������������λ������ʱ��2�֣����ʱ�����֣����ʱ��1�֣�����6�֡�������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ
��H2O����4����������һ������DZ����������������Ǽ���ij��������������λ������ʱ��2�֣����ʱ�����֣����ʱ��1�֣�����6�֡�������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ ��
��
��ϰ��ϵ�д�
�����Ŀ


 ��
��
 ��
��
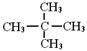
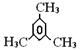
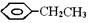
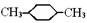
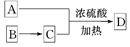 ��ʾת����ϵ��D�ķ���ʽΪC10H12O3��A��C��Ӧ�Ļ�ѧ����ʽΪ______________________________________________________��
��ʾת����ϵ��D�ķ���ʽΪC10H12O3��A��C��Ӧ�Ļ�ѧ����ʽΪ______________________________________________________��