��Ŀ����
��һ���㡢Ϻ����������С�ӱߣ������ε����������������������ס��ҡ��������������ų��ķ�Һ�ÿ��ֻ����Na2CO3��FeCl3��Ca(OH)2��HCl�е�һ�֡�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壻�ܶ����������ݣ���ˮ���塣
������������⣺
(1)���������ų��ķ�Һ�ﺬ�е���Ⱦ�����ǣ�
��______________����______________����____________����____________��
(2)�ڶ�������ij��ȡ���ĺ�ˮ���϶����е�������______________________��
(3)С�����㡢Ϻ����������ԭ����____________________________________
���𰸡�
(1)Ca(OH)2��FeCl3��HCl��Na2CO3
(2)Na����Ca2����Fe3����H����Cl��
(3)��ˮ������Լ��к���ѧҩƷ�ĸ���������ֱ��Σ���㡢Ϻ�����������
�����������⿼��Ԫ�ػ������֪ʶ����϶Ժ�ˮ�ļ���������ҳ�ʲô��ѧ�������������������ʣ���ˮ�������������Ⱦ��Ȼ��ɼ����жϣ��ó����

��ϰ��ϵ�д�
�����Ŀ
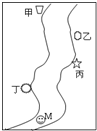 ��ͼ��ʾ����һ���㡢Ϻ����������С�ӱߣ������ε����������мס��ҡ����������������������ŷŵķ�ˮ�ﶼֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壻�ܶ����������ݣ���ˮ�Գ��壮��ش�
��ͼ��ʾ����һ���㡢Ϻ����������С�ӱߣ������ε����������мס��ҡ����������������������ŷŵķ�ˮ�ﶼֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壻�ܶ����������ݣ���ˮ�Գ��壮��ش�