��Ŀ����
������ͭˮ��Һ����μ��백ˮ�����γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ��������Һ�м����Ҵ�������ɫ���壨��ѧʽΪ[Cu(NH3)4]SO4��H2O��������
(1)д������ʵ��ǰ������Ӧ�����ӷ���ʽ__________��____________��
(2)ͭԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________________��ͭ���ʾ����е�ԭ��ѻ�ģ������_________�ѻ�����ѻ�ģ�����ƣ���
(3)����������ɫ���������ķǽ���Ԫ���У��縺��������_________����Ԫ�ط��ţ�����һ������������_________����Ԫ�ط��ţ����þ����е������ӵ����幹����_________�������ӵ�����ԭ�ӵ��ӻ���ʽΪ_________��
(4)���ķе�_________������ڡ����ڡ����(PH2)��ԭ����_____________��
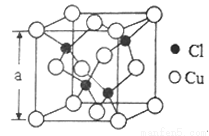
(5)Cu��һ���Ȼ��ᄃ���ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��______________�����þ�����ܶ�Ϊpg��cm-3����NA��ʾ����٤����������þ����ı߳�Ϊa=_____________nm��
һ���¶��£������������Ϊ0.5 L�ĺ����ܱ������з�����Ӧ��
CO(g)+Cl2(g) COCl2(g)
COCl2(g)
������������5 minʱ����ƽ�⡣
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | ||
CO | Cl2 | COCl2 | COCl2 | ||
�� | 500 | 1.0 | 1.0 | 0 | 0.8 |
�� | 500 | 1.0 | a | 0 | 0.5 |
�� | 600 | 0.5 | 0.5 | 0.5 | 0.7 |
����˵����ȷ����
A. ��������ǰ5 min��ƽ����Ӧ����v(CO)=0.16 mol��L-1��min-1
B. �÷�Ӧ������ӦΪ���ȷ�Ӧ
C. ����������ʼʱCl2�����ʵ���Ϊ0.55 mol
D. ����ʼʱ���������м���CO 0.8 mol��Cl2 0.8 mol���ﵽƽ��ʱCO��ת���ʴ���80%



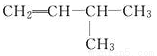 2-��-3-��ϩ
2-��-3-��ϩ CCH3 2-��Ȳ
CCH3 2-��Ȳ 1��5-���ױ�
1��5-���ױ�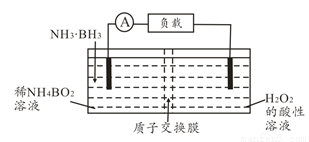
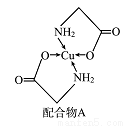

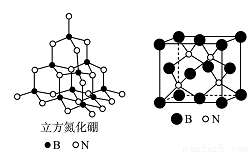
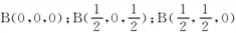 �ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��
�ȡ��������������Bԭ������ҵȾ��Nԭ�ӵ��������Ϊ____________________ ��