��Ŀ����
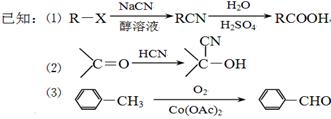
�㶹����һ����;�㷺�����ϣ����������Ҵ���B(����ʽΪC7H6O2)ͨ������;���ϳɡ�
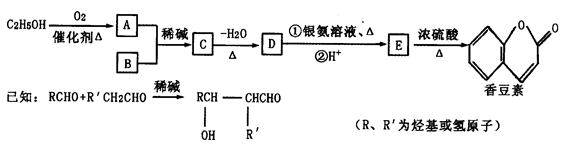
��1��D��E�Тٵķ�Ӧ����Ϊ________________________________��
C�к��������ŵ�����Ϊ_________________________________��
��2���й��㶹�ص�˵����ȷ����___________________________(����ĸ)��
a��������ˮ�;ƾ���
b�����ڷ����㶹�أ�������������
c���ܷ����ӳɷ�Ӧ�����ܷ���ȡ����Ӧ
d��1mol�㶹�ؿ���2molNaOH��Ӧ
��3��B�ж���ͬ���칹�壬���б�����ֻ��һ��������ͬ���칹��Ľṹ��ʽ��________________(дһ�ּ���)��
��4���Ҵ�����A�Ļ�ѧ����ʽΪ__________________________��

��1��D��E�Тٵķ�Ӧ����Ϊ________________________________��
C�к��������ŵ�����Ϊ_________________________________��
��2���й��㶹�ص�˵����ȷ����___________________________(����ĸ)��
a��������ˮ�;ƾ���
b�����ڷ����㶹�أ�������������
c���ܷ����ӳɷ�Ӧ�����ܷ���ȡ����Ӧ
d��1mol�㶹�ؿ���2molNaOH��Ӧ
��3��B�ж���ͬ���칹�壬���б�����ֻ��һ��������ͬ���칹��Ľṹ��ʽ��________________(дһ�ּ���)��
��4���Ҵ�����A�Ļ�ѧ����ʽΪ__________________________��
��8�֣���1��������Ӧ��1�֣����Ȼ����ǻ���1�֣� ��2��bd��2�֣�
��3��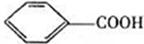 ��
��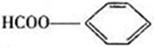 ��2�֣�
��2�֣�
��4��2CH3CH2OH��O2 2CH3CHO��2H2O��2��
2CH3CHO��2H2O��2��
��3��
 ��
�� ��2�֣�
��2�֣���4��2CH3CH2OH��O2
 2CH3CHO��2H2O��2��
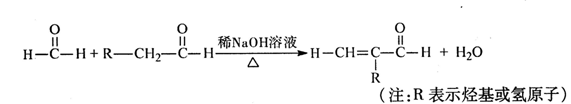
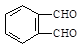
2CH3CHO��2H2O��2������������Ҵ��ڴ����������£�����������Ӧ������ȩ�����A����ȩ���ṹ��ʽ��CH3CHO��D�ܷ���������Ӧ��˵��D�к���ȩ����E����������������Ӧ�������㶹�أ�������㶹�صĽṹ��ʽ��֪��E�Ľṹ��ʽӦ����
 ������D�Ľṹ��ʽ��
������D�Ľṹ��ʽ�� ��������֪��Ϣ��֪��B�Ľṹ��ʽ��
��������֪��Ϣ��֪��B�Ľṹ��ʽ��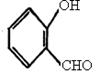 ������C�Ľṹ��ʽ��
������C�Ľṹ��ʽ�� ��
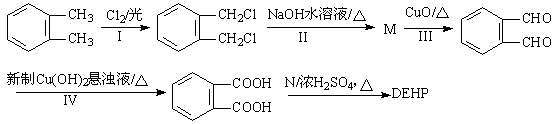
����1���������Ϸ�����֪��D����E��ȩ����������Ӧ������C�Ľṹ��ʽ��֪��C�����к��еĹ��������ǻ����Ȼ���
��2��a���㶹���к���������������ˮ�������ھƾ��У�a����ȷ��b���㶹���к��з��ǻ������ױ����������Գ��ڷ����㶹�أ������������ʣ�b��ȷ�� c���㶹���еı�����̼̼˫���ܷ����ӳɷ�Ӧ�������ͱ������Է���ȡ����Ӧ��c����ȷ��d����������ˮ����ֲ���1�����ǻ�������1mol�㶹�ؿ���2molNaOH��Ӧ��d��ȷ����ѡbd��
��3������B�Ľṹ��ʽ��֪��������ֻ��һ��������B��ͬ���칹��Ľṹ��ʽ��
 ��
�� ��
����4���Ҵ�����������������ȩ����Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH��O2
 2CH3CHO��2H2O��
2CH3CHO��2H2O��
��ϰ��ϵ�д�
�����Ŀ
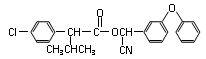 ) ��
) ��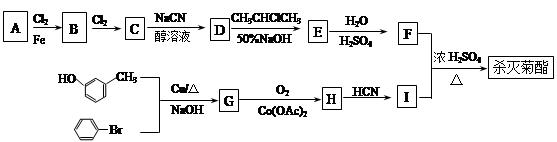

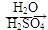 J��д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ�� ___________________��
J��д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ�� ___________________��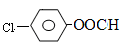 �ṹ������ϴ�������X���� �֡�
�ṹ������ϴ�������X���� �֡� 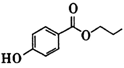 ���ѱ����������ල��������ֽܾ�ֹ��ΪʳƷ���Ӽ�ʹ�ã����й�������˵��������� ��
���ѱ����������ල��������ֽܾ�ֹ��ΪʳƷ���Ӽ�ʹ�ã����й�������˵��������� ��

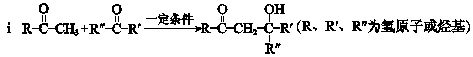
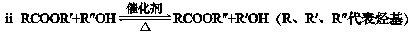
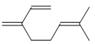 ������Ҫ�Ļ���ԭ�ϣ��㷺����������ҵ��
������Ҫ�Ļ���ԭ�ϣ��㷺����������ҵ��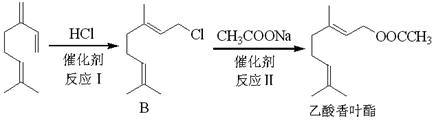

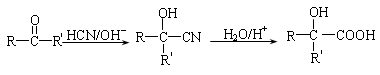

 ��XΪ±ԭ�ӣ�MΪ������������ȡ������)
��XΪ±ԭ�ӣ�MΪ������������ȡ������)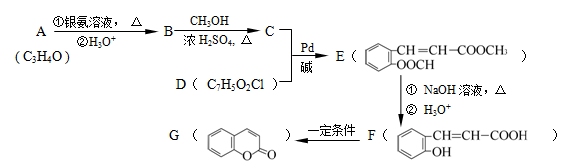
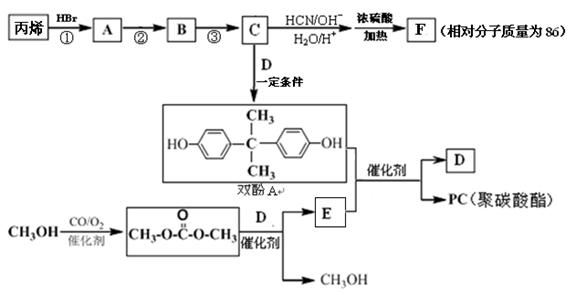
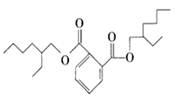
 ���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨
���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨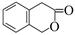 ����Ҫ�õ����Լ��У�NaOH��Һ��������
����Ҫ�õ����Լ��У�NaOH��Һ��������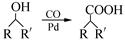 (R��R��Ϊ����)����д���Ա��ͱ�ϩ��
(R��R��Ϊ����)����д���Ա��ͱ�ϩ��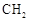 =CH��CH3��
=CH��CH3��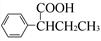 ��·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
��·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�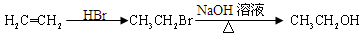
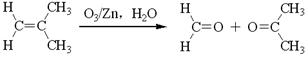
 ��������H2��Ӧ����������H2 mol��
��������H2��Ӧ����������H2 mol��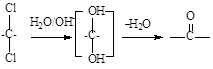 �ķ�Ӧ�õ�
�ķ�Ӧ�õ�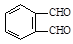 ����д����ͬ���칹��Ľṹ��ʽ�� ��
����д����ͬ���칹��Ľṹ��ʽ�� ��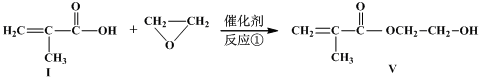
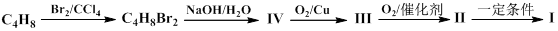
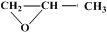 �������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ��
�������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ��