��Ŀ����
��֪MgCl2��6H2O�����ڿ����м���ʱ���ͷŲ��ֽᾧˮ��ͬʱ����Mg��OH��Cl����ʽ�Ȼ�þ��������MgO�������ǹ���MgCl2��6H2O���ۺ����ã�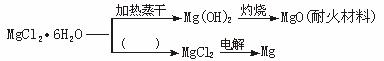
��ش��������⣺
��1����ͼ�е���������д�ʵ��ķ�Ӧ������
��2��Mg��OH��2������������ܽ�ƽ�⣺
Mg��OH��2��s��![]() Mg2����2OH��������ϵ�м��루���������ֲ�ͬ�������ʣ�______________��������Mg��OH��2�ܽ⡣
Mg2����2OH��������ϵ�м��루���������ֲ�ͬ�������ʣ�______________��������Mg��OH��2�ܽ⡣
��3��NaF��MgO�ĺ˼����ֱ�Ϊ2.31��10��10 m��2.10��10��10 m�������ߵ��۵�ֱ�Ϊ993 ���2852 �档�Խ�������ܵ�ԭ��
��4��������ɫ��ѧ��ԭ�Ӿ��õĸ������ѧ��Ӧ��ԭ����ÿ��ԭ�Ӷ����뷴Ӧ��ȫ��ת��Ϊ�����203 kg MgCl2��6H2Oԭ�ϣ����Ի��28.8 kg MgO��________kg 36.5%�������________kg MgCl2��
������n��MgO����![]() ��745 mol n��MgCl2����
��745 mol n��MgCl2����![]() ��745��255 mol
��745��255 mol
��m��MgCl2����255��95��24225 g��24.225 kg
�������غ㣺36.5%������������203��29.8��24.2��149 kg��
�𰸣���1���ڸ�����Ȼ��������м���
��2�����ࣺHCl��H2SO4��HNO3�ȣ�ǿ�������Σ�CuSO4��FeCl3�ȣ�����ࣺCH3COONH4��NH4Cl�ȣ�ֻҪд�����༴�ɣ�
��3����������ͬ�����Ӿ��壬MgO��NaF�����̣���MgO��NaF�˼����С����Mg2����O2������������ࣨ����֮�����üӴ����Ӿ�����۵�Ҳ�������ߡ�
��4��149 24.2

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
