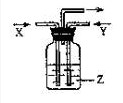
��Ŀ����
��14�֣���ͼ��ʾװ�������������Ʊ������������֤��β�������IJ�������װ�ã����ȼ��г̶ֹ�װ�þ�����ȥ�������������Ҫ��ش����⡣
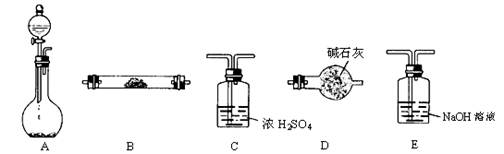
��1������ƿ��ʢװZnƬ����Һ©����ʢװϡH2SO4����
�ٵ��Ʊ�H2����֤H2�Ļ�ԭ�Բ�����H2�����������������˳��Ϊ:A��C��B
��B��Dʱ������ʹ��Bװ�ã�������ʢ��ҩƷ������ ��
��д��ѧʽ����Dװ�õ�������
��
������ʵ����Ϊ��ʹH2���������ʲ����ڹ��죬�ڲ��ı�����ҩƷ�������£��ɲ�ȡ�ķ����� ��
����Ҫ��д�������ַ���ԭ����ͬ��
��2������ƿ��ʢװNa2O2����Һ©����ʢװŨ��ˮ����
����ƿ�ڳ����ܲ���O2�⣬���ܲ�������NH3���ܲ�������NH3��ԭ���ǣ�
a ��b ��c ��
���ò��������������Ĵ�����ʵ�飬������װ�ð������������ң�����˳���ǣ�A�� �� �� ������ȷ��ŵ���ĸ����ʱ�����е�һ��װ���п��ܻ���ִ�������ɫ���壬��װ���� ������ĸ��ʾ�����û�ѧ����ʽ���ͺ���ɫ���������ԭ�� ��
��

��1������ƿ��ʢװZnƬ����Һ©����ʢװϡH2SO4����
�ٵ��Ʊ�H2����֤H2�Ļ�ԭ�Բ�����H2�����������������˳��Ϊ:A��C��B
��B��Dʱ������ʹ��Bװ�ã�������ʢ��ҩƷ������ ��
��д��ѧʽ����Dװ�õ�������
��
������ʵ����Ϊ��ʹH2���������ʲ����ڹ��죬�ڲ��ı�����ҩƷ�������£��ɲ�ȡ�ķ����� ��
����Ҫ��д�������ַ���ԭ����ͬ��
��2������ƿ��ʢװNa2O2����Һ©����ʢװŨ��ˮ����
����ƿ�ڳ����ܲ���O2�⣬���ܲ�������NH3���ܲ�������NH3��ԭ���ǣ�
a ��b ��c ��
���ò��������������Ĵ�����ʵ�飬������װ�ð������������ң�����˳���ǣ�A�� �� �� ������ȷ��ŵ���ĸ����ʱ�����е�һ��װ���п��ܻ���ִ�������ɫ���壬��װ���� ������ĸ��ʾ�����û�ѧ����ʽ���ͺ���ɫ���������ԭ�� ��
��
��14�֣�����ʽ2�֣�����ÿ��1�֣���1���� CuO�� CuSO4�� ��ֹ�����е�ˮ����װ�ã�����H2��������ļ��顣
�ڿ��Ʒ�Һ©���Ļ�����ʹϡH2SO4�������£� ��ˮ������ƿ�����ˮ�л��ñ�ë��������ƿ���������ƹ���ȣ���������������ɸ��֣�
��2����Na2O2��ˮ��Ӧ���ȣ�����NH3���ܽ�ȣ���ʹNH3��H2O�����ֽ⣩��
Na2O2��ˮ��Ӧ����ˮ����ʹˮ�����٣�������NH3���ܽ����� Na2O2��ˮ��Ӧ����OH�C��c��OH�C����������NH3��H2O�ĵ���������NH3���ݳ���
��A��D��B��E����D����˳������÷֣��� B
4NH3+5O2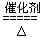 4NO+6H2O �� 2NO+O2===2NO2
4NO+6H2O �� 2NO+O2===2NO2
�ڿ��Ʒ�Һ©���Ļ�����ʹϡH2SO4�������£� ��ˮ������ƿ�����ˮ�л��ñ�ë��������ƿ���������ƹ���ȣ���������������ɸ��֣�
��2����Na2O2��ˮ��Ӧ���ȣ�����NH3���ܽ�ȣ���ʹNH3��H2O�����ֽ⣩��
Na2O2��ˮ��Ӧ����ˮ����ʹˮ�����٣�������NH3���ܽ����� Na2O2��ˮ��Ӧ����OH�C��c��OH�C����������NH3��H2O�ĵ���������NH3���ݳ���
��A��D��B��E����D����˳������÷֣��� B
4NH3+5O2
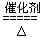 4NO+6H2O �� 2NO+O2===2NO2
4NO+6H2O �� 2NO+O2===2NO2��

��ϰ��ϵ�д�
 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ