��Ŀ����
Ϊ�ⶨij��Ѫ����Ʒ����Ҫ�ɷ���������������(FeSO4��7H2O)������Ԫ�صĺ�����ij��ѧ��ȤС�����������ʵ�鷽��:
����һ:������KMnO4��Һ�ζ��ⶨ��Ԫ�صĺ���
��д���ζ���Ӧ�����ӷ���ʽ ��
(2)���еζ���ʽ�У���������� (�г�������ȥ)(����ĸ��š�
(3)ʵ��ǰ������Ҫȷ����һ�����ʵ���Ũ�ȵ�����KMnO4��Һ250mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_ (����������)��
(4)��ijͬѧȡ5Ƭ��Ѫ����Ʒ���100 mL��Һ��ȡ20. 00 mL��������Ũ��Ϊc1mol��L-1��KMnO4��Һ�ζ�����ȥV1 mL����ÿƬ��Ѫ������Ԫ�ص�����_ g(�ô���ʽ��ʾ)��
������:��FeSO4ת��ΪFe2O3���ⶨ�����仯��������������:

(5)���������H2O2��Ŀ����_ _��
(6)�������һϵ�в���������:���ˡ�ϴ�ӡ�_ ����ȴ��������
(7)����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ_ g(�ú�a�Ĵ���ʽ��ʾ)��
(8)��ijͬѧ�����ԭ��ص���ʽʵ��Fe2+��Fe3+��ת��������ͨ��O2���������ҺΪϡ���ᣬ��д�������ĵ缫��Ӧʽ ��
����һ:������KMnO4��Һ�ζ��ⶨ��Ԫ�صĺ���
��д���ζ���Ӧ�����ӷ���ʽ ��
(2)���еζ���ʽ�У���������� (�г�������ȥ)(����ĸ��š�

(3)ʵ��ǰ������Ҫȷ����һ�����ʵ���Ũ�ȵ�����KMnO4��Һ250mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_ (����������)��
(4)��ijͬѧȡ5Ƭ��Ѫ����Ʒ���100 mL��Һ��ȡ20. 00 mL��������Ũ��Ϊc1mol��L-1��KMnO4��Һ�ζ�����ȥV1 mL����ÿƬ��Ѫ������Ԫ�ص�����_ g(�ô���ʽ��ʾ)��
������:��FeSO4ת��ΪFe2O3���ⶨ�����仯��������������:

(5)���������H2O2��Ŀ����_ _��
(6)�������һϵ�в���������:���ˡ�ϴ�ӡ�_ ����ȴ��������
(7)����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ_ g(�ú�a�Ĵ���ʽ��ʾ)��
(8)��ijͬѧ�����ԭ��ص���ʽʵ��Fe2+��Fe3+��ת��������ͨ��O2���������ҺΪϡ���ᣬ��д�������ĵ缫��Ӧʽ ��
��15�֣�1��5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O ��2�֣� ��2��B ��1�֣�
��3��50mL����ƿ��2�֣��������0�֣� ��4��0.28c1v1��2�֣�
��5����Fe2��������Fe3����2�֣� ��6������ ��1�֣� ��7��0.07a ��2�֣�
��8��O2��4e����4H����2H2O��2�֣�
��3��50mL����ƿ��2�֣��������0�֣� ��4��0.28c1v1��2�֣�
��5����Fe2��������Fe3����2�֣� ��6������ ��1�֣� ��7��0.07a ��2�֣�
��8��O2��4e����4H����2H2O��2�֣�
�����������1�����Ը�����ؾ���ǿ�����ԣ���Fe2+����ΪFe3+��MnO4������ԭΪMn2+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
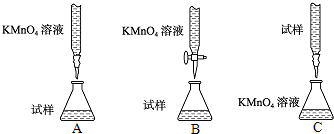
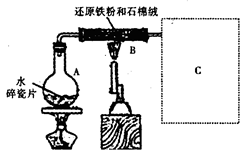
��2�����Ը�����ؾ���ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧʢ������ʽ�ζ����ڣ�����������Һ�����ԣ�Ӧʢ������ʽ�ζ����ڣ�װ��A��C�о��Ǽ�ʽ�ζ��ܣ�����B����ʡ�
��3����ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ�250mL����ƿ��Զ�̻�ȱ��250mL����ƿ��
��4�����ĸ�����ص����ʵ�����0.001c1v1mol�����ݷ���ʽ��֪���������ӵ����ʵ�����0.005c1v1mol����5Ƭ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol��
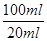 ��0.025c1v1mol������ÿƬ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol�����ÿƬ��Ѫ������Ԫ�ص�������0.005c1v1mol��56g/mol��0.28c1v1g��
��0.025c1v1mol������ÿƬ��Ѫ����Ʒ���������ӵ����ʵ�����0.005c1v1mol�����ÿƬ��Ѫ������Ԫ�ص�������0.005c1v1mol��56g/mol��0.28c1v1g����5��Ҫ�����������������������ӣ�����Ҫ������������˫��ˮ�dz��õ���ɫ�����������˫��ˮ�������ǽ�Fe2��������Fe3����
��6���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ������������Ȼ���������ɵ�����������ȴ�������������������
��7��������ԭ���غ��֪��ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������Ԫ�ص�����Ϊ
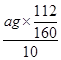 ��0.07ag��
��0.07ag����8��ԭ����������õ����ӣ�������ԭ��Ӧ������������ͨ�룬����Һ�����ԣ����������缫��ӦʽΪO2��4e����4H����2H2O��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

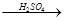 H2
H2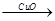 Cu����CuO
Cu����CuO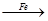 Cu
Cu