��Ŀ����
п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������Zn��NH3��42+���ش��������⣺
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ___________���û�ѧʽ��ʾ����
��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ��___________________��
��3�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������______________��
�� ���������������� �� ����п���������� ���������Ͱ�ˮ �� ����п�Ͱ�ˮ
��4���ڻ���͵��������У���������ʯī��TiO2��һ��������Ͼ��ȣ�Ϳ�ڽ������棬�ڸ��������գ�������������TiC����һ�����µ����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
��5��ij����� ������
������ ��
�� ��
�� ����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��

�ݴ˻ش��������⣺
������� ��
�� �Ļ�ѧʽ��B________��D__________��
�Ļ�ѧʽ��B________��D__________��
��д��D��ϡ���ᷴӦ�Ļ�ѧ����ʽ��__________________��
д����Ӧ�ٵ����ӷ���ʽ��___________________��
��ϰ��ϵ�д�
�����Ŀ




 ��
��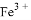 ��
��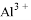 ���������ӣ��������м��������
���������ӣ��������м��������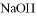 ��Һ���Ȳ����裬���ˣ����������ټ�����������ᣬ������Һ�д������ӵ��������ǣ� ��
��Һ���Ȳ����裬���ˣ����������ټ�����������ᣬ������Һ�д������ӵ��������ǣ� ��

 2Pb2++2H2O
2Pb2++2H2O CH3CH2OH(g)+H2O(g) ��H=-256.1kJ/mol
CH3CH2OH(g)+H2O(g) ��H=-256.1kJ/mol