��Ŀ����
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4+��Cl����Mg2����Ba2����CO32����SO42������ȡ����100 mL��Һ��������ʵ�飺
(1)��һ�ݼ���AgNO3��Һ�г���������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol��
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
��������ʵ�飬�����Ʋ���ȷ����(����)
��K��һ������
��100 mL��Һ�к�0.01 mol CO32��
��Cl�����ܴ���
��Ba2��һ��������
��Mg2�����ܴ���
(1)��һ�ݼ���AgNO3��Һ�г���������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol��
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
��������ʵ�飬�����Ʋ���ȷ����(����)
��K��һ������
��100 mL��Һ�к�0.01 mol CO32��
��Cl�����ܴ���
��Ba2��һ��������
��Mg2�����ܴ���
| A���ۢܢ� | B���٢ۢ� | C���٢� | D���ۢ� |
B
��(2)֪����NH4+ 0.04 mol����(3)֪����CO32�� 0.02 mol������SO42�� 0.01 mol�����Բ�����Mg2����Ba2����������Һ�ʵ�����K�����ٺ���0.02 mol����Cl���Ƿ�������жϡ�
�㲦�����⿼�����ӵļ��飬���鿼�������Ӽ�����ж��������Ѷ��еȡ�
�㲦�����⿼�����ӵļ��飬���鿼�������Ӽ�����ж��������Ѷ��еȡ�

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 mol��L��1
mol��L��1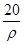 mol��L��1
mol��L��1 mol��L��1
mol��L��1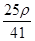 mol��L��1
mol��L��1


 ��
��