��Ŀ����
I��ijͬѧ���ʵ�齫Fe3O4��CuO��ɵĻ����X������ͼ��ʾ��ת����ÿһ������Ӧ��ȫ��

��1����������B�Ļ�ѧ����ʽ��____________________________��
��2��������DͶ�����ϡ���Ტ��ַ�Ӧ���ˣ�������Һ����˫��ˮ���û�ɫ��Һ�� д��������˫��ˮʱ��Һ�з�����Ӧ�����ӷ���ʽ��________________________ ��
��3������֪����X������Ϊ7��2g��Ӧ��Al�۵�������2��7g������B�ڱ�״���µ����Ϊ672mL�������D��������____________ g��
II�������A��̼���ƺ�̼��������ɡ���������������ȵĻ����A��
��4����������һ������100mL 3mol/L��Ba(OH)2��Һ����������ʹHCO3-��CO32-ȫ����Ϊ���������˺�������Һ�м���200mL 2mol/L��ϡ���ᣬ��Һǡ�ó����ԡ�
��д�����з�Ӧ�����ӷ���ʽ
̼��������������������ķ�Ӧ��____________________________��
�ڻ����A��NaHCO3������Ϊ____________________g��
��5��������һ�����ȼ�50mL8mol/L��HCl��Һ��ʹHCO3-��CO32-ȫ����ΪCO2���ټ�50mL2mol/L��
Ba(OH)2��Һ����Ϻ����ҺpH=14����Ϻ���Һ����仯���Բ��ơ��ڳ����£��������A��Na2CO3������Ϊ_______________g��
��2��������DͶ�����ϡ���Ტ��ַ�Ӧ���ˣ�������Һ����˫��ˮ���û�ɫ��Һ�� д��������˫��ˮʱ��Һ�з�����Ӧ�����ӷ���ʽ��________________________ ��
��3������֪����X������Ϊ7��2g��Ӧ��Al�۵�������2��7g������B�ڱ�״���µ����Ϊ672mL�������D��������____________ g��
II�������A��̼���ƺ�̼��������ɡ���������������ȵĻ����A��
��4����������һ������100mL 3mol/L��Ba(OH)2��Һ����������ʹHCO3-��CO32-ȫ����Ϊ���������˺�������Һ�м���200mL 2mol/L��ϡ���ᣬ��Һǡ�ó����ԡ�
��д�����з�Ӧ�����ӷ���ʽ
̼��������������������ķ�Ӧ��____________________________��
�ڻ����A��NaHCO3������Ϊ____________________g��
��5��������һ�����ȼ�50mL8mol/L��HCl��Һ��ʹHCO3-��CO32-ȫ����ΪCO2���ټ�50mL2mol/L��
Ba(OH)2��Һ����Ϻ����ҺpH=14����Ϻ���Һ����仯���Բ��ơ��ڳ����£��������A��Na2CO3������Ϊ_______________g��
��1��2Al+2NaOH+2H2O==2NaAlO2+3H2��
��2��2Fe2++H2O2+2H+==2Fe2++2H2O
��3��5.28
��4���� ����16.8g
����16.8g
��5��5.3g
��2��2Fe2++H2O2+2H+==2Fe2++2H2O
��3��5.28
��4����
 ����16.8g
����16.8g��5��5.3g

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
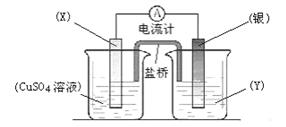
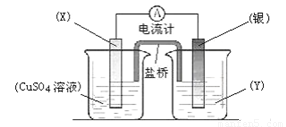
 ����һ��____________________________________��
����һ��____________________________________��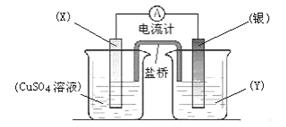
 ����һ��____________________________________��
����һ��____________________________________��