��Ŀ����
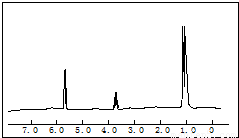
 5.8gij��������������������������ȫȼ�գ��������ĸ�����������ͨ��Ũ���������������Һ��ʹŨ��������5.4g������������Һ����13.2g���Ը����ʽ����������������õ�����ͼ�����ʺɱȷֱ��27��29��31��44��50��58�������ߣ�ͼ�Ǹ����ʵĺ˴Ź������ף����ҷ�����ȷֱ�Ϊ1��1��6������������Ϣ������������⣺
5.8gij��������������������������ȫȼ�գ��������ĸ�����������ͨ��Ũ���������������Һ��ʹŨ��������5.4g������������Һ����13.2g���Ը����ʽ����������������õ�����ͼ�����ʺɱȷֱ��27��29��31��44��50��58�������ߣ�ͼ�Ǹ����ʵĺ˴Ź������ף����ҷ�����ȷֱ�Ϊ1��1��6������������Ϣ������������⣺��1��ȷ�������ʵķ���ʽ��
��2���Ʋ���ṹ��д�������ʵĽṹ��ʽ��������
���������ʺɱȿ�֪���л������Է�������Ϊ58������8g�л�������ʵ���Ϊ
=0.1mol��
Ũ��������5.4g��ΪH2O����������n��H��=2��
=0.6mol��
����������Һ����13.2g��ΪCO2����������n��C��=
=0.3mol��
����1mol�л����к���3molC��6molH����������Ϊ3��12+6=42g����Ӧ��OԪ�ص�����Ϊ58g-42g=16g����ȷ���л���ķ���ʽ�����ݺ˴Ź�������֪���������ʲ�ͬ��H����ԭ�Ӹ�����Ϊ1��1��6���Դ�ȷ���ṹ��ʽ��
| 5.8g |
| 58g/mol |
Ũ��������5.4g��ΪH2O����������n��H��=2��
| 5.4g |
| 18g/mol |
����������Һ����13.2g��ΪCO2����������n��C��=
| 13.2g |
| 44g/mol |
����1mol�л����к���3molC��6molH����������Ϊ3��12+6=42g����Ӧ��OԪ�ص�����Ϊ58g-42g=16g����ȷ���л���ķ���ʽ�����ݺ˴Ź�������֪���������ʲ�ͬ��H����ԭ�Ӹ�����Ϊ1��1��6���Դ�ȷ���ṹ��ʽ��
����⣺��1�����ʺɱȿ�֪���л������Է�������Ϊ58������8g�л�������ʵ���Ϊ
=0.1mol��
Ũ��������5.4g��ΪH2O����������n��H��=2��
=0.6mol��
����������Һ����13.2g��ΪCO2����������n��C��=
=0.3mol��
����1mol�л����к���3molC��6molH����������Ϊ3��12+6=42g����Ӧ��OԪ�ص�����Ϊ58g-42g=16g��
��1mol������n��O��=
=1mol�����Է���ʽΪC3H6O��
�𣺸����ʵķ���ʽΪC3H6O��
��2�����ݺ˴Ź�������֪���������ʲ�ͬ��H����ԭ�Ӹ�����Ϊ1��1��6����ṹ��ʽӦΪCH3CHOHCH3��Ϊ2-������
�𣺸����ʵĽṹ��ʽΪCH3CHOHCH3������Ϊ2-������
| 5.8g |
| 58g/mol |
Ũ��������5.4g��ΪH2O����������n��H��=2��
| 5.4g |
| 18g/mol |
����������Һ����13.2g��ΪCO2����������n��C��=
| 13.2g |
| 44g/mol |
����1mol�л����к���3molC��6molH����������Ϊ3��12+6=42g����Ӧ��OԪ�ص�����Ϊ58g-42g=16g��
��1mol������n��O��=
| 16g |
| 16g/mol |
�𣺸����ʵķ���ʽΪC3H6O��
��2�����ݺ˴Ź�������֪���������ʲ�ͬ��H����ԭ�Ӹ�����Ϊ1��1��6����ṹ��ʽӦΪCH3CHOHCH3��Ϊ2-������
�𣺸����ʵĽṹ��ʽΪCH3CHOHCH3������Ϊ2-������
���������⿼���л������ʽ��ȷ������Ŀ�Ѷ��еȣ�����ע���ʺɱ��Լ��˴Ź�������Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ