��Ŀ����
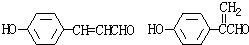
��10�֣�X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81��8����
��1��1��Y������Ӧ���� ����ԭ�ӡ�
��2��X�ķ���ʽ�� ��
��
��3��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
��д��A�Ľṹ��ʽ ��
��E�� F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��
��ѧ����ʽ��ͬ���칹��ÿ��2�֣�����һ��1��
����

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д���10�֣�X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81��8����
��1��1��Y������Ӧ���� ����ԭ�ӡ�
��2��X�ķ���ʽ�� ��
��3��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
 |
��д��A�Ľṹ��ʽ ��
��E��F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��


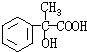
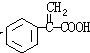 +H2O
+H2O