��Ŀ����
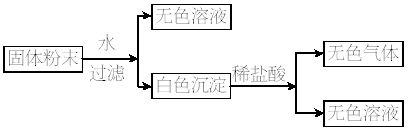
һ�������ĩ������������NaCl��CuSO4��Na2O2��K2CO3����NH4��2SO4��Na2SO4��KNO3�����������е�ij���ֻ�϶��ɡ�ijͬѧ�������������²�����Ȼ�������ͼ���ȷ����������ɺ��ɷֵ����ʵ�������.��ȡ
��.������������Һ�ֳ����ȷݣ�һ�ݼ����̪��Һ�ʺ�ɫ����Ũ��Ϊ4.0 mol��L-1?�������кͣ���ȥ������12.5 mL������һ����ϡ�����ữ��������������ټ��������Ba��NO3��2��Һ���ð�ɫ����������Ϊ
��.���������õ�
��1�����Է�����������ʵ�������������ݵļ��㣩����ȷ���÷�ĩ��һ�������е���__________��һ�����е���__________��
��2���������㣺Ϊ�˽�һ��ȷ���÷�ĩ�������ɷ��Ƿ���ڣ���ͬѧ�ֶ��й����ݽ����˴����������յó���������ɺ��ɷֵ����ʵ�������д�����ļ�����̡�
��1��CuSO4��K2CO3��NaCl��Na2O2����NH4��2SO4
��2���ɶ��Է���֪��Na2O2����NH4��2SO4һ�����У�Na2SO4��KNO3���ܺ���
��
��2Na2O2+2H2O====4NaOH+O2��
x 2x x/2
2NaOH+��NH4��2SO4====Na2SO4+2NH3��+2H2O
2y y 2y
���ԣ�![]() +2y=
+2y=![]() =0.15 mol��
=0.15 mol��
������֪��������Ӧ��NaOH������������NaOH�������кͣ�
���ԣ�2x-2y=2��0.012
�ۺϣ����x=0.1 mol��y=0.05 mol
����Һ�ֳ����ȷݺ���һ���м��������Ba��NO3��2��Һ����������BaSO4�����ʵ���Ϊ��![]() =0.055 mol
=0.055 mol
0.1 mol��
![]() =0.1 mol
=0.1 mol
����
��������ĩ��������ˮ����ɫ��Һ��˵����CuSO4���������壬����ͨ��Ũ���ᣬ����������Լ�С��˵����������NH3����ͨ����ʯ�ң�����������䣬˵����O2���Ӷ�˵��ԭ��Ʒһ������NH4��2SO4��Na2O2���������ữ�����������˵����Na2CO3����Ba��NO3��2���ټ���AgNO3����������˵����NaCl��Na2SO4��KNO3���жϡ�
��2�����𰸡�