��Ŀ����
��ҵ����������β���ŷų���SO2��NOx�ȣ����γ���������Ҫ���أ������ɿ����еĻҳ������ᡢ���ᡢ�л�̼�⻯����������γɵ���������1��SO2���̳��Ĵ����γ�����ķ�Ӧ����ʽ��
��2����֪2SO2��g��+O2��g��?2SO3��g����H=-196kJ/mol����߷�Ӧ��SO2��ת���ʣ��Ǽ���SO2�ŷŵ���Ч��ʩ��
��T�¶�ʱ����2L�ݻ��̶�������ܱ������м���2.0mol SO2��1.0mol O2��5min��Ӧ�ﵽƽ�⣬���������ת����Ϊ50%����ԣ�O2��=
���ڢٵ������£��жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��
a��SO2��O2��SO3���ߵ�Ũ��֮��Ϊ2��1��2 b�������������ѹǿ����
c�������ڻ��������ܶȱ��ֲ��� d��SO3�����ʵ������ٱ仯
e��SO2���������ʺ�SO3�������������
��3�������е�SO2������NaOH��Һ���գ������õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ�����缫����Ϊʯī��
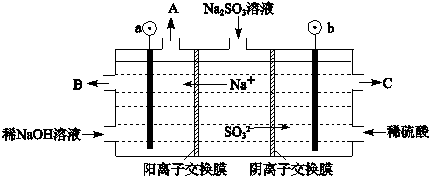
��ͼ��a��Ҫ���ӵ�Դ�ģ������������
��SO32-�ŵ�ĵ缫��ӦʽΪ
�۵�������������12.6g Na2SO3�����������仯������Ϊ
��������1��SO2���̳��Ĵ����γ�������2SO2+2H2O+O2=2H2SO4��
��2���ٸ�������ʽ���ת�������ٸ��ݷ�Ӧ���ʵĶ���ʽ���㣻
�ڻ�ѧƽ��ı�־�����淴Ӧ������ͬ������ֺ������ֲ��䣻
��3���ٸ��ݵ������������ӵ��ƶ������������������������жϢ�ͼ��a��Ҫ���ӵ�Դ�ĸ�����SO32-������ʧȥ���ӱ��SO42-������C��������������H2SO4����SO32-ʧȥ���ӱ�������SO42-��
�۵��������������������ˮ��Ϊ�������ƣ��������仯�����������������Ƶ�������ȥ����ˮ��������
���ݵ����غ���㣺
2e-��Na2SO3����2H2O��2NaOH���������仯������
126g 36g 80g 44g
12.6g x
x=4.4g
��2���ٸ�������ʽ���ת�������ٸ��ݷ�Ӧ���ʵĶ���ʽ���㣻
�ڻ�ѧƽ��ı�־�����淴Ӧ������ͬ������ֺ������ֲ��䣻
��3���ٸ��ݵ������������ӵ��ƶ������������������������жϢ�ͼ��a��Ҫ���ӵ�Դ�ĸ�����SO32-������ʧȥ���ӱ��SO42-������C��������������H2SO4����SO32-ʧȥ���ӱ�������SO42-��
�۵��������������������ˮ��Ϊ�������ƣ��������仯�����������������Ƶ�������ȥ����ˮ��������
���ݵ����غ���㣺
2e-��Na2SO3����2H2O��2NaOH���������仯������
126g 36g 80g 44g
12.6g x
x=4.4g
����⣺��1��SO2���̳��Ĵ����γ�������2SO2+2H2O+O2=2H2SO4���ʴ�Ϊ��2SO2+2H2O+O2=2H2SO4��
��2����T1�¶�ʱ����2L���ܱ������м���4.0molSO2��2.0molO2��5min��Ӧ�ﵽƽ�⣬���������ת����Ϊ50%��
2SO2��g��+O2?2SO3��g����H��0
��ʼ����mol�� 2.0 1.0 0
�仯����mol��2.0��50% 0.5 0.5
ƽ������mol�� 1.0 0.5 0.5
ǰ5min��O2��ƽ����Ӧ����=
=0.05mol/��L?min����
�ʴ�Ϊ��0.05mol/��L?min����
�ڻ�ѧƽ��ı�־�����淴Ӧ������ͬ������ֺ������ֲ��䣻
a�����ʵ�Ũ�ȹ�ϵ����ʼ����ת�����йأ�SO2��O2��SO3���ߵ�Ũ��֮��Ϊ2��1��2 ����˵����Ӧ�ﵽƽ��״̬����a�����ϣ�
b����Ϊ�÷�ӦΪ�������ʵ�������ķ�Ӧ�����º��������£�ѹǿΪ�������������������ѹǿ����˵���ﵽƽ��״̬����b���ϣ�
c����Ӧǰ�������������䣬����������䣬�ܶ��ڷ�Ӧ�����к�ƽ��״̬�����䣬�����л��������ܶȱ��ֲ��䲻��˵�����ôﵽƽ��״̬����c�����ϣ�
d��SO3�����ʵ������ٱ仯����˵����Ӧ�ﵽƽ��״̬����d���ϣ�
e��SO2���������ʺ�SO3�������������˵�����淴Ӧ������ͬ����˵����Ӧ�ﵽƽ��״̬����e���ϣ�
�ʴ�Ϊ��bde��
��3���ٸ��ݵ������������ӵ��ƶ������������������������жϢ�ͼ��a��Ҫ���ӵ�Դ�ĸ�����SO32-������ʧȥ���ӱ��SO42-������C��������������H2SO4��
�ʴ�Ϊ���������
��SO32-ʧȥ���ӱ�������SO42-���缫��ӦʽΪ��SO32--2e-+H2O=SO42-+2H+���ʴ�Ϊ��SO32--2e-+H2O=SO42-+2H+��
�۵��������������������ˮ��Ϊ�������ƣ��������仯�����������������Ƶ�������ȥ����ˮ��������
���ݵ����غ㣺
2e-��Na2SO3����2H2O��2NaOH���������仯������
126g 36g 80g 44g
12.6g x
x=4.4g
������12.6g Na2SO3�����������仯������Ϊ4.4 g���ʴ�Ϊ��4.4g��
��2����T1�¶�ʱ����2L���ܱ������м���4.0molSO2��2.0molO2��5min��Ӧ�ﵽƽ�⣬���������ת����Ϊ50%��
2SO2��g��+O2?2SO3��g����H��0
��ʼ����mol�� 2.0 1.0 0
�仯����mol��2.0��50% 0.5 0.5
ƽ������mol�� 1.0 0.5 0.5
ǰ5min��O2��ƽ����Ӧ����=
| 0.5mol |
| 2L?5min |
�ʴ�Ϊ��0.05mol/��L?min����
�ڻ�ѧƽ��ı�־�����淴Ӧ������ͬ������ֺ������ֲ��䣻
a�����ʵ�Ũ�ȹ�ϵ����ʼ����ת�����йأ�SO2��O2��SO3���ߵ�Ũ��֮��Ϊ2��1��2 ����˵����Ӧ�ﵽƽ��״̬����a�����ϣ�
b����Ϊ�÷�ӦΪ�������ʵ�������ķ�Ӧ�����º��������£�ѹǿΪ�������������������ѹǿ����˵���ﵽƽ��״̬����b���ϣ�
c����Ӧǰ�������������䣬����������䣬�ܶ��ڷ�Ӧ�����к�ƽ��״̬�����䣬�����л��������ܶȱ��ֲ��䲻��˵�����ôﵽƽ��״̬����c�����ϣ�
d��SO3�����ʵ������ٱ仯����˵����Ӧ�ﵽƽ��״̬����d���ϣ�
e��SO2���������ʺ�SO3�������������˵�����淴Ӧ������ͬ����˵����Ӧ�ﵽƽ��״̬����e���ϣ�
�ʴ�Ϊ��bde��
��3���ٸ��ݵ������������ӵ��ƶ������������������������жϢ�ͼ��a��Ҫ���ӵ�Դ�ĸ�����SO32-������ʧȥ���ӱ��SO42-������C��������������H2SO4��
�ʴ�Ϊ���������
��SO32-ʧȥ���ӱ�������SO42-���缫��ӦʽΪ��SO32--2e-+H2O=SO42-+2H+���ʴ�Ϊ��SO32--2e-+H2O=SO42-+2H+��
�۵��������������������ˮ��Ϊ�������ƣ��������仯�����������������Ƶ�������ȥ����ˮ��������
���ݵ����غ㣺
2e-��Na2SO3����2H2O��2NaOH���������仯������
126g 36g 80g 44g
12.6g x
x=4.4g
������12.6g Na2SO3�����������仯������Ϊ4.4 g���ʴ�Ϊ��4.4g��
���������⿼���˻�ѧ��Ӧ���ʵļ��㡢��ѧƽ����жϡ��绯ѧ�ȣ�������ض�ѧ������֪ʶ��ѵ���ͼ��飬���������ѧ�����������ѧ֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ
�±��Ǽ׳���ij�տ����������棺
����ijУ�о���ѧϰС��Ա�����Ҫ��Ⱦ��SO2��������ij������̽����
ʵ��һ������ͼ��ʾװ�ý���ʵ�飮

��Aװ�õ������� ����������������SO2���壮
��ʵ������У�Bװ����ʯ����ֽ����ɫû�з����仯��Cװ����ʪ�����ɫʯ����ֽ�� ɫ��˵��SO2��ˮ��Ӧ����һ���ᣬ����Ļ�ѧʽ�� ��
��Dװ�õ������� ����ʵ������װ����ͨ���״����2.24L SO2���壬��NaOH��Һ���������������Na2SO3�����ʵ���Ϊ mol������Ӧ�Ļ�ѧ����ʽΪ��SO2+2NaOH=Na2SO3+H2O��
ʵ�������ʢ��ˮ���ձ���ͨ��SO2���壬���������Һ���� �ԣ����ᡱ������С�����Ȼ��ÿ��1h�ⶨ��pH������pH��С��ֱ���㶨��˵���ձ�����Һ�������е�����������������H2SO4�� SO2�γ��������һ;���� SO2������е�O2��Ʈ���������·�Ӧ����SO3��SO3���ڽ�ˮ����H2SO4���ڴ˹�����Ʈ���� ���������������������
SO2������е�������ˮ��Ӧ����������γ����꣮���п����׳������꣮
��1��������ɵ�Σ���ǣ� ����һ������
��2�������ŷŵ�β�������ᡢ���ʵȹ�ҵ�����ų��ķ����ж����е����������������������ˮ����ת��Ϊ ��������������һ��Ҫԭ��
������������������Ϊ�˼�������β����ɵ���Ⱦ�������������ƹ�ʹ�û�������ͣ����������а�һ���������� ���ˮ�����Ҵ�������
| ���� | ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ | ��������״�� |
| �� | 55 | SO2 | II | �� |
ʵ��һ������ͼ��ʾװ�ý���ʵ�飮

��Aװ�õ�������
��ʵ������У�Bװ����ʯ����ֽ����ɫû�з����仯��Cװ����ʪ�����ɫʯ����ֽ��
��Dװ�õ�������
ʵ�������ʢ��ˮ���ձ���ͨ��SO2���壬���������Һ����
��1��������ɵ�Σ���ǣ�
��2�������ŷŵ�β�������ᡢ���ʵȹ�ҵ�����ų��ķ����ж����е����������������������ˮ����ת��Ϊ
������������������Ϊ�˼�������β����ɵ���Ⱦ�������������ƹ�ʹ�û�������ͣ����������а�һ����������
��ҵ����������β���ų��ĵ���������ǿ�������Ҫ��ȾԴ��Ϊ������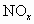 ����Ⱦ����ͨ������������
����Ⱦ����ͨ������������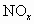 ��ԭΪ������
��ԭΪ������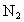 ��
��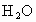 ������
������
����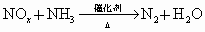
�������к���������NO��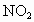 �Ļ������3.0L����ͬ��ͬѹ�µ�3.5L
�Ļ������3.0L����ͬ��ͬѹ�µ�3.5L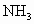 ǡ��ʹ�û��������ȫ��Ӧת��Ϊ
ǡ��ʹ�û��������ȫ��Ӧת��Ϊ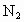 ����������NO��
����������NO��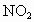 ���������
���������
[����]
|
A��1:4 |
B��1:3 |
|
C��1:2 |
D��1:1 |
��12�֣��±��Ǽ׳���ij�տ����������棺
| ���� | ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ | ��������״�� |
| �� | 55 | SO2 | II | �� |
��̽��ʵ�顿
ʵ��һ������ͼ��ʾװ�ý���ʵ�顣
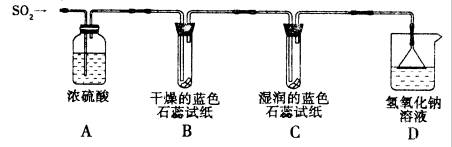
��Aװ�õ������� ����������������SO2���塣
��ʵ������У�Bװ����ʯ����ֽ����ɫû�з����仯��Cװ����ʪ�����ɫʯ����ֽ������ɫ��˵��SO2��ˮ��Ӧ����һ���ᣬ����Ļ�ѧʽ�� ��
��Dװ�õ������� ����ʵ������װ����ͨ���״����2��24L SO2���壬��NaOH��Һ���������������Na2SO3�����ʵ���Ϊ mol������Ӧ�Ļ�ѧ����ʽΪ��SO2��2NaOH��Na2SO3��H2O��
ʵ�������ʢ��ˮ���ձ���ͨ��SO2���壬���������Һ���� �ԣ����ᡱ������С�����Ȼ��ÿ��1 h�ⶨ��pH������pH��С��ֱ���㶨��˵���ձ�����Һ�������е�����������������H2SO4��
���������ϡ�SO2�γ��������һ;��; SO2������е�O2��Ʈ���������·�Ӧ����SO3��
SO3���ڽ�ˮ����H2SO4���ڴ˹�����Ʈ���� ���������������������
��̽�����ۡ�SO2������е�������ˮ��Ӧ����������γ����ꡣ���п����׳������ꡣ
��֪ʶ���롿
��1��������ɵ�Σ���ǣ� ����һ������
��2�������ŷŵ�β�������ᡢ���ʵȹ�ҵ�����ų��ķ����ж����е����������������������ˮ����ת��Ϊ ��������������һ��Ҫԭ��
��������顿������������������Ϊ�˼�������β����ɵ���Ⱦ�������������ƹ�ʹ�û�������ͣ����������а�һ���������� ���ˮ�����Ҵ�������
 ��ҵ�����У�����۵ķ�Ӧ����Ϊ
��
��ҵ�����У�����۵ķ�Ӧ����Ϊ
��