��Ŀ����
��Ԫ�����ڱ��������ɵ�֪ʶ�ش��������⣺
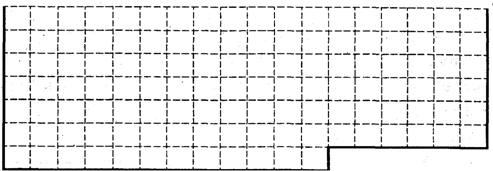
��1��������ͼ��ʾԪ�����ڱ�����ʵ����ǰ36��Ԫ�����ڵķ�Χ�ı߽磮
��2�����бȽ�Ԫ�����ʵĵݱ������ȷ����______��
a�����Ӱ뾶��Na+��Mg2+��S2-��Cl-b�����ԣ�HClO4��H2SO4��H3PO4��H4SiO4
c���⻯���ȶ��ԣ�H2O��H2S��PH3��SiH4d��NH3���H+��������H2O��
��3��Ԫ�ؼ��ǵ������ڢ�A��Ԫ�أ���Ԫ���������ַǽ���Ԫ�ؿ�������ӻ�����A��д��A�ĵ���ʽ______����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����д���û�ѧ��Ӧ�Ļ�ѧ����ʽ______��
��4�����������ǽϻ��õķǽ���Ԫ�أ���һ����ѧ����ʽ˵�����ֵ��ʵ�������ǿ��______��
��5��������Ԫ�ص���̬�⻯���У�Ԫ���ҵ��⻯�����ȶ���д��Ԫ���ҵĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ______��
��6��0.1mol�ĵ���Cl2��100mL1.5mol/L��FeBr2��Һ��Ӧ����������Fe2+��Br-�����ʵ���֮����______��
��1��������ͼ��ʾԪ�����ڱ�����ʵ����ǰ36��Ԫ�����ڵķ�Χ�ı߽磮

��2�����бȽ�Ԫ�����ʵĵݱ������ȷ����______��
a�����Ӱ뾶��Na+��Mg2+��S2-��Cl-b�����ԣ�HClO4��H2SO4��H3PO4��H4SiO4
c���⻯���ȶ��ԣ�H2O��H2S��PH3��SiH4d��NH3���H+��������H2O��
��3��Ԫ�ؼ��ǵ������ڢ�A��Ԫ�أ���Ԫ���������ַǽ���Ԫ�ؿ�������ӻ�����A��д��A�ĵ���ʽ______����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����д���û�ѧ��Ӧ�Ļ�ѧ����ʽ______��
��4�����������ǽϻ��õķǽ���Ԫ�أ���һ����ѧ����ʽ˵�����ֵ��ʵ�������ǿ��______��
��5��������Ԫ�ص���̬�⻯���У�Ԫ���ҵ��⻯�����ȶ���д��Ԫ���ҵĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ______��
��6��0.1mol�ĵ���Cl2��100mL1.5mol/L��FeBr2��Һ��Ӧ����������Fe2+��Br-�����ʵ���֮����______��
��1��ǰ36��Ԫ��Ϊһ��������Ԫ�أ�36��Ԫ��Ϊ봣�����ʵ����ǰ36��Ԫ�����ڵķ�Χ�ı߽磬��ͼΪ
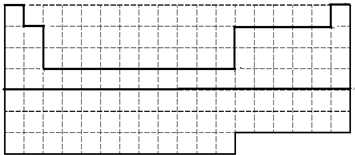 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��a�����ӵĵ��Ӳ�Խ�࣬�뾶Խ������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶ΪS2-��Cl-��Na+��Mg2+����a����
b���ǽ�����ΪCl��S��P��Si��������������ˮ��������ΪHClO4��H2SO4��H3PO4��H4SiO4����b��ȷ��
c���ǽ�����ΪO��S��P��Si����̬�⻯���ȶ���ΪH2O��H2S��PH3��SiH4����c��ȷ��
d����������⻯���������ӵ�����ǿ����NH3���H+��������H2Oǿ����d����
�ʴ�Ϊ��bc��
��3��Ԫ�ؼ��ǵ������ڢ�A��Ԫ�أ�ΪClԪ�أ��������ַǽ���Ԫ�ؿ�������ӻ�����A����AΪNH4Cl�������ʽΪ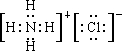 ����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����������������ᣬ�÷�ӦΪ2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����������������ᣬ�÷�ӦΪ2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
�ʴ�Ϊ��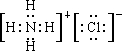 ��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
��4���ǽ�����O��N�������õõ���������֤ͬ������4NH3+5O2
2N2+6H2O��4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
2N2+6H2O��4NH3+5O2
4NO+6H2O��
��5������Ԫ�ص���̬�⻯���У�Ԫ���ҵ��⻯�����ȶ�����ΪF���䵥����ˮ��Ӧ����HF���������÷�ӦΪ2F2+2H2O�T4HF+O2���ʴ�Ϊ��2F2+2H2O�T4HF+O2��
��6��0.1mol�ĵ���Cl2ʧȥ0.2mol���ӣ�100mL1.5mol/L��FeBr2��Һ��n��FeBr2��=0.15mol���������ӻ�ԭ��ǿ���������ӿ�ȫ�����������豻������������Ϊx���ɵ����غ��֪��0.2mol=0.15mol+x����1-0�������x=0.05mol�����Ա�������Fe2+��Br-�����ʵ���֮����0.15mol��0.05mol=3��1���ʴ�Ϊ��3��1��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��a�����ӵĵ��Ӳ�Խ�࣬�뾶Խ������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶ΪS2-��Cl-��Na+��Mg2+����a����
b���ǽ�����ΪCl��S��P��Si��������������ˮ��������ΪHClO4��H2SO4��H3PO4��H4SiO4����b��ȷ��
c���ǽ�����ΪO��S��P��Si����̬�⻯���ȶ���ΪH2O��H2S��PH3��SiH4����c��ȷ��
d����������⻯���������ӵ�����ǿ����NH3���H+��������H2Oǿ����d����
�ʴ�Ϊ��bc��
��3��Ԫ�ؼ��ǵ������ڢ�A��Ԫ�أ�ΪClԪ�أ��������ַǽ���Ԫ�ؿ�������ӻ�����A����AΪNH4Cl�������ʽΪ
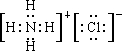 ����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����������������ᣬ�÷�ӦΪ2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
����ҵ�Ͽ���MnSO4��Һ�������������Mn2O3����������������ᣬ�÷�ӦΪ2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl���ʴ�Ϊ��
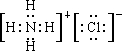 ��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl��
��2MnSO4+Cl2+3H2O�TMn2O3+2H2SO4+2HCl����4���ǽ�����O��N�������õõ���������֤ͬ������4NH3+5O2
| ||
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| ||
| �� |
��5������Ԫ�ص���̬�⻯���У�Ԫ���ҵ��⻯�����ȶ�����ΪF���䵥����ˮ��Ӧ����HF���������÷�ӦΪ2F2+2H2O�T4HF+O2���ʴ�Ϊ��2F2+2H2O�T4HF+O2��
��6��0.1mol�ĵ���Cl2ʧȥ0.2mol���ӣ�100mL1.5mol/L��FeBr2��Һ��n��FeBr2��=0.15mol���������ӻ�ԭ��ǿ���������ӿ�ȫ�����������豻������������Ϊx���ɵ����غ��֪��0.2mol=0.15mol+x����1-0�������x=0.05mol�����Ա�������Fe2+��Br-�����ʵ���֮����0.15mol��0.05mol=3��1���ʴ�Ϊ��3��1��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ