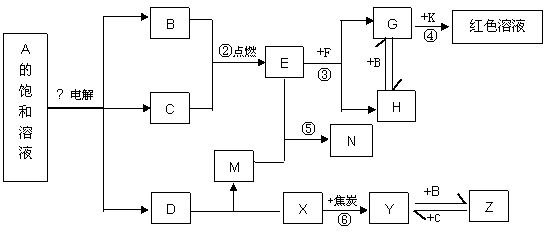
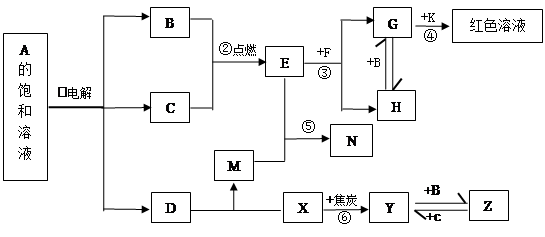��Ŀ����
�ڳ���ʱ����V1 mL C1 mol/L CH3COOH��Һ�еμӵ�V2 mL C2 mol/L NaOH��Һ�У����н����У�������ǣ�������
������A�����ݵ������Ũ�Ȼ��ʱ��������������ǡ����ȫ��Ӧ������Һ�е�����ֻ�д����ƣ��ɴ�������ӵ�ˮ����������
B����pH=7����֪c��OH-��=c��H+���������õ���غ���������
C�������Һ��pH��7��������Һ�е�������������
D������������ʱ�������Һ��pH��7������Һ�����ԣ�Ӧ��֤�������������
B����pH=7����֪c��OH-��=c��H+���������õ���غ���������
C�������Һ��pH��7��������Һ�е�������������
D������������ʱ�������Һ��pH��7������Һ�����ԣ�Ӧ��֤�������������
����⣺A��V1=V2��C1=C2�����������������ǡ����ȫ��Ӧ����Ӧ����Һ�е�����ֻ�д����ƣ��ɴ�������ӵ�ˮ����c��Na+����c��CH3COO-������A����
B��pH=7����c��OH-��=c��H+�����ɵ���غ�c��OH-��+c��CH3COO-��=c��Na+��+c��H+����֪��c��CH3COO-��=c��Na+������B��ȷ��
C�������Һ��pH��7����Һ�е����ʿ���Ϊ�����ƻ���������������ƵĻ�����Ϊ�����ƣ���V1C1=V2C2����C��ȷ��
D��V1=V2���һ����Һ��pH��7������Һ�����ԣ���Һ�е�����Ϊ����ʹ����ƣ���C1��C2����D��ȷ��
��ѡA��
B��pH=7����c��OH-��=c��H+�����ɵ���غ�c��OH-��+c��CH3COO-��=c��Na+��+c��H+����֪��c��CH3COO-��=c��Na+������B��ȷ��
C�������Һ��pH��7����Һ�е����ʿ���Ϊ�����ƻ���������������ƵĻ�����Ϊ�����ƣ���V1C1=V2C2����C��ȷ��
D��V1=V2���һ����Һ��pH��7������Һ�����ԣ���Һ�е�����Ϊ����ʹ����ƣ���C1��C2����D��ȷ��
��ѡA��
���������⿼���������Һ�Ķ��Է��������ӵ�Ũ�ȹ�ϵ����ȷ����Ϻ���Һ�е����ʴ������ֿ��ܵ�����ǽ��Ĺؼ�����ע������������ʵĵ�����ε�ˮ�������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ