��Ŀ����
��9�֣���0.6 molͭͶ��100mLһ��Ũ�ȵ�HNO3���������У�����HNO3��Ũ�Ȳ�ͬ�����ܷ���������Ӧ������ÿ����Ӧ�в���������ֻ��һ�֣���
��1����֪������Ӧ�ШD����Ӧ�����ӷ���ʽ����Ӧ�ڣ���д�����D����Ӧ����Ӧ�٣������ӷ���ʽ��
��____________________________________________________________________��
��3Cu+8H++2NO3�� ===== 3Cu2++2NO��+4H2O��
��2������ֻ������Ӧ�٣������������ڱ�״���µ����Ϊ_____L������ֻ������Ӧ�ڣ������������ڱ�״���µ����Ϊ________L��
��3��ʵ�ʲ����������ڱ�״���µ����Ϊ22.4 L����μӷ�Ӧ�ٵ�ͭ��μӷ�Ӧ�ڵ�ͭ�����ʵ���֮��Ϊ___________��
��1��Cu+4H++2NO3�� ==== Cu2++2NO2��+2H2O��2�֣�
��2��26.88��8.96����2�֣�
��3��3��1��3�֣�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д������ܡ�����δ�������������Դ��
��.ʵ���ã�1g����ȼ������Һ̬ˮʱ�ų�142.9kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ_______��������ţ�
A��2H2��g��+O2��g��  2H2O��l����H= ��142.9kJ��mol��1 2H2O��l����H= ��142.9kJ��mol��1 |
B��H2��g��+1/2 O2��g��  H2O��l����H= ��285.8kJ��mol��1 H2O��l����H= ��285.8kJ��mol��1 |
C��2H2+O2 2H2O��l����H= ��571.6kJ��mol��1 2H2O��l����H= ��571.6kJ��mol��1 |
D��H2��g��+1/2 O2��g��  H2O��g�� ��H= ��285.8kJ��mol��1 H2O��g�� ��H= ��285.8kJ��mol��1 |
��CaBr2+H2O
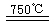 CaO+2HBr ��2HBr+Hg
CaO+2HBr ��2HBr+Hg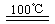 HgBr2+H2
HgBr2+H2��HgBr2+_____
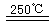 ______________ ��2HgO
______________ ��2HgO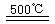 2Hg+O2��
2Hg+O2��������ݡ�ԭ�Ӿ��á���˼�������������۵Ļ�ѧ����ʽ��____________��
���ݡ���ɫ��ѧ����˼�������÷�����H2����Ҫȱ�㣺______________��
��.���ú��ܰ�ˮ�ֽ�����������Ŀǰ�����о��Ŀ��⡣��ͼ�����е�һ�����̣��������˹����ĵ⡣����ʾ����Ӧ�ڵIJ�����O2��SO2��H2O��
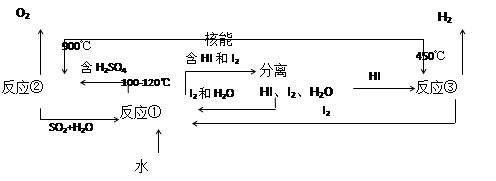
������з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��__________________________����Ӧ��__________________________��
�˷���ȡ����������ŵ���_______________________________________________��
��.����ͨ��������ˮú���ķ����Ƶá�����CO��g��+ H2O��g��
 CO2��g��+ H2��g��; ��H<0��
CO2��g��+ H2��g��; ��H<0����850��ʱ��K=1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK______1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ���� 1.0 mol CO��3.0molH2O��1.0mol CO2�� x mol H2����
�ٵ�x=5.0ʱ������ƽ����___________��������Ӧ���淴Ӧ��������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������__________��
��3����850��ʱ������x��5.0 mol��x��6.0mol���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ����H2����������ֱ�Ϊa����b������a _______ b������ڡ�����С�ڡ����ڡ�����
��.������ԭ����ͭ���õĺ�ɫ���������ͭ��������ͭ�Ļ�����֪Cu2O��������Һ�пɷ�������������ԭ��Ӧ������Cu2+�͵���ͭ��
��1������8������ͭ��������ԭ�õ���ɫ����6.8�ˣ����к�����ͭ��������ͭ�����ʵ���֮���� ��
��2������6.8�������������������ϡ�����ַ�Ӧ����ˣ��ɵõ����� g��
��3������6.8�������������һ������Ũ�����ַ�Ӧ��
�����ɱ�״����1.568�������壨������NO2���ܽ⣬Ҳ������NO2��N2O4��ת�������������ijɷ��� �������ʵ���֮���� ��
�ڰѵõ�����ҺС������Ũ�����������ľ�����ˣ��þ���23.68g����������ԭ��Һ�е�Cu2+��20%������ĸҺ�С������þ���Ļ�ѧʽ
Ŀǰ������̼���������ܹ�ע��CO2�IJ�������Ч�������ó�Ϊ��ѧ���о�����Ҫ���⡣��������ѧ֪ʶ������������⣺
��1����֪ij��Ӧ��ƽ�����ʽΪ�� ������Ӧ�Ļ�ѧ��ӦΪ��__? ___
������Ӧ�Ļ�ѧ��ӦΪ��__? ___
��2����������������C(s)��H2O(g)�ֱ����ס��������ܱ���������������1���з�Ӧ��������������±���ʾ��
���� | �ݻ�/L | �¶�/�� | ��ʼ��/mol | ƽ����/mol[ | �ﵽƽ������ʱ��/min | |
C(s) | H2O(g) | H2(g) | ||||
�� | 2 | T1 | 2 | 4 | 3.2 | 8 |
�� | 1 | T2 | 1 | 2 | 1,2 | 3 |
��T10Cʱ���÷�Ӧ��ƽ�ⳣ��K=_______
���������У�����Ӧ���е�1.5minʱ��H2O(g)�����ʵ���Ũ��_______ (��ѡ����ĸ����
A��=0.8 mol��L-1??? B��=1.4 mol��L-1??? C��<1.4 mol��L-1??? D��>1.4 mol��L-1
�����������ݻ�Ϊ1L��T1��ʱ����������ȳ���C(s)��H2O(g)��CO2(g)��H2(g)�� �ﵽƽ?? ��ʱ�����������������������ȫ��ͬ����_______(��ѡ����ĸ����
A.0.6 mol��1.0 mol��0.5 mol��1.0 mol??
B�� 0.6 mol��2.0 mol��0 mol��0 mol
C.1.0 mol��2.0 mol��1.0 mol��2.0 mol??????
D�� 0.25 mol��0.5 mol��0.75 mol��1.5 mol
��3����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״�����֪CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5kJ/mol����H����285.8kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ��?????????????????????????????? ��
��4����ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��
2CO2(g��+ 6H2(g�� CH3OCH3(g��+ 3H2O(g)
CH3OCH3(g��+ 3H2O(g)
��֪һ��ѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ��±���
Ͷ�ϱ�[n(H2��/ n(CO2)] | 500 K | 600 K | 700 K | 800 K |
1.5 | 45% | 33% | 20% | 12% |
2.0 | 60% | 43% | 28% | 15% |
3.0 | 83% | 62% | 37% | 22% |
���÷�Ӧ���ʱ���H?? 0���ر���S___0�����������
���ü�����Ϊȼ�ϵ��ԭ�ϣ��ڼ��Խ����иõ�ظ����ĵ缫��Ӧʽ?????????????? ������1.12 L��min-1����״������������õ����ͨ����ѣ��е�Ϊ-24.9 �������øõ�ص��500 mL 2 mol��L-1 CuSO4��Һ��ͨ��0.50 min�������Ͽ���������ͭ???????? g��
 2Hg+O2��
2Hg+O2��
 2H2O��l��
��H= ��142.9kJ��mol��1
2H2O��l��
��H= ��142.9kJ��mol��1 CaO+2HBr ��2HBr+Hg
CaO+2HBr ��2HBr+Hg HgBr2+H2
HgBr2+H2 ______________
��2HgO
______________
��2HgO 2Hg+O2��
2Hg+O2��
 CO2��g��+ H2��g��; ��H<0��
CO2��g��+ H2��g��; ��H<0�� CaO+2HBr
CaO+2HBr HgBr2+H2
HgBr2+H2  ______
______  Hg+O2��
Hg+O2�� 
 CO2(g)+H2(g) ��H<0 ��850��ʱ��
CO2(g)+H2(g) ��H<0 ��850��ʱ��