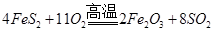��Ŀ����
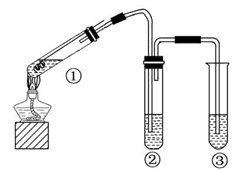
�������ֳƻ�������Ҫ�ɷ�FeS2�����ʲ�����Ԫ�أ����ǵؿ��зֲ������������dz�Ƶ���ɫ�������Ľ�������������Ϊ�ǻƽ𣬹��ֳơ����˽𡱡�
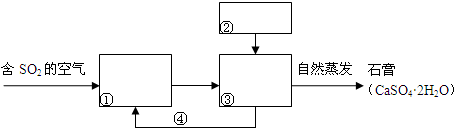
��ҵ����������Ҫ���ڽӴ����������ᣬ�䷴Ӧԭ��Ϊ��
��FeS2��O2�����·�Ӧ����SO2��
��SO2�ڴ�����������O2��Ӧ����SO3��
��SO3��H2O��Ӧ����H2SO4��
��1����1.00�� SO2��O2�Ļ�������к�SO2 0.40�֣���һ�������·�����Ӧ�ڣ���80%��SO2����ת������Ӧ����������SO3������������_________��
��2������80�������������ᣬ���������100��98%�����ᡣ����Ӧ��������Ԫ�ص���ʧ��Ϊ5%������������FeS2����������Ϊ__________________��
��3����Ũ�����м����ܽ�SO3�����γɵ�Һ��Ʒ������ᣬ��Ũ��ͨ���������SO3��������ʾ����20%�ķ������ἴ��ʾ�������к���20%��SO3������1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮԼ���٣���д��������̣�
��4����֪��850�桫900��ʱ�������������������գ����ܷ������з�Ӧ��
��3FeS2��8O2��Fe3O4��6SO2
��4FeS2��11O2��2Fe2O3��8SO2
ΪʹFeS2������ȫ����Fe2O3����ҵ��ʹ�ù�������������������20%ʱ��������¯����SO2�������������д��������̣�
57����480 g������FeS2����������ȫ��Ӧ�������ù�����n��Fe����n��O����4��a����ʱ��������b mol����д��b��a�Ĺ�ϵʽ_______________________��
��ҵ����������Ҫ���ڽӴ����������ᣬ�䷴Ӧԭ��Ϊ��
��FeS2��O2�����·�Ӧ����SO2��
��SO2�ڴ�����������O2��Ӧ����SO3��
��SO3��H2O��Ӧ����H2SO4��
��1����1.00�� SO2��O2�Ļ�������к�SO2 0.40�֣���һ�������·�����Ӧ�ڣ���80%��SO2����ת������Ӧ����������SO3������������_________��
��2������80�������������ᣬ���������100��98%�����ᡣ����Ӧ��������Ԫ�ص���ʧ��Ϊ5%������������FeS2����������Ϊ__________________��
��3����Ũ�����м����ܽ�SO3�����γɵ�Һ��Ʒ������ᣬ��Ũ��ͨ���������SO3��������ʾ����20%�ķ������ἴ��ʾ�������к���20%��SO3������1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮԼ���٣���д��������̣�
��4����֪��850�桫900��ʱ�������������������գ����ܷ������з�Ӧ��
��3FeS2��8O2��Fe3O4��6SO2
��4FeS2��11O2��2Fe2O3��8SO2
ΪʹFeS2������ȫ����Fe2O3����ҵ��ʹ�ù�������������������20%ʱ��������¯����SO2�������������д��������̣�
57����480 g������FeS2����������ȫ��Ӧ�������ù�����n��Fe����n��O����4��a����ʱ��������b mol����д��b��a�Ĺ�ϵʽ_______________________��
��1��0.4��40%40%
��2��78.9%
��3��241.2g
��4��78.4%
57��b��0.5a��8
��2��78.9%
��3��241.2g
��4��78.4%
57��b��0.5a��8
�����������1��2SO2+O2
 2SO3��n��SO2������n��SO3��. ������Ӧ��SO2����m��SO2��= 0.40�֡�80%=0.32�֣��������SO3������Ϊ80/64��0.32��=0.4�֡��ʷ�Ӧ����������SO3������������0.4��1��100%=0.4��40%��
2SO3��n��SO2������n��SO3��. ������Ӧ��SO2����m��SO2��= 0.40�֡�80%=0.32�֣��������SO3������Ϊ80/64��0.32��=0.4�֡��ʷ�Ӧ����������SO3������������0.4��1��100%=0.4��40%����2������Ԫ���غ��֪��ϵʽΪFeS2����2SO2����2SO3����2H2SO4��1mol FeS2����ȡ2mol H2SO4.��120��FeS2����ȫת������ȡ196�ִ��������ᡣ�������������FeS2����������ΪX������б���ʽ120:�� 2��98�� =80��X��1-5%��:�� 100��98%��.���X=78.9%��
��3������1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮԼ���٣�m����Һ��= 1.95g/cm3��1000ml ="1950g" .m��SO3��= 1.95g/cm3��1000ml��30%=1950g="585" g.��SO3��������������Ϊ585 g��98/80=716.6g. m��H2SO4��= 1.95g/cm3��1000ml����1-30%��=1365g����1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮ����ΪY����1950g+Y�� �� 95%=716.6g+1365g.���Y=241.2g��
��4��4FeS2��11O2
 2Fe2O3��8SO2 ���跴Ӧ����8Ħ����SO2������������Ҫ11Ħ����O2��������������20%������ʵ��ͨ������������ʵ���Ϊ11��120%mol =13.2mol.����������Ϊ2.2mol.��ͬ��ͬѹ�£�����������˵���������Ⱦ������ǵ����ʵ����ıȡ�������¯����SO2���������8�£�8+2.2����100%=78.4%��
2Fe2O3��8SO2 ���跴Ӧ����8Ħ����SO2������������Ҫ11Ħ����O2��������������20%������ʵ��ͨ������������ʵ���Ϊ11��120%mol =13.2mol.����������Ϊ2.2mol.��ͬ��ͬѹ�£�����������˵���������Ⱦ������ǵ����ʵ����ıȡ�������¯����SO2���������8�£�8+2.2����100%=78.4%��57����FeS2��n��Fe��:n��S��=1:2��480g FeS2�����ʵ���Ϊ480g��120g/mol=4mol.n��SO2��=8mol.n��Fe��=4mol�������ù�����n��Fe����n��O����4��a�� n��Fe��=4mol�����ڹ����е���ԭ�ӵ����ʵ���Ϊamol.�������Ĺ����й����ĵ����������ʵ���Ϊb��0.5a��8��

��ϰ��ϵ�д�
�����Ŀ