��Ŀ����
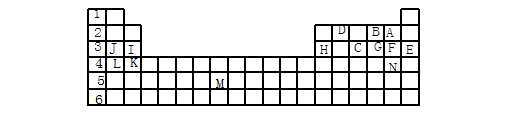
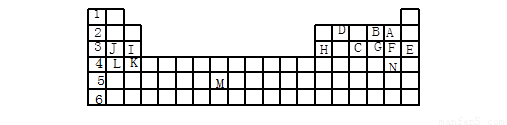
(12��)�±��������Ԫ�����ڱ���һ����Ԫ�أ��ش���������(ÿ��1��)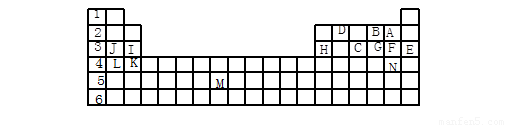
(1) ��������ĸ�����15��Ԫ���У���ѧ��������õ��� ����Ԫ�ط��ű�ʾ����ͬ������������ǿ���� ���ǽ�������ǿ���� �������µ���ΪҺ̬�ķǽ���Ԫ���� ����������Ԫ�ص��� ���ڹ���Ԫ�ص��� ���ÿ�����ĸ��ʾ����
(2) B��F��C��̬�⻯��Ļ�ѧʽ�ֱ�Ϊ �� �� �������� ��ȶ���
(3) ����������ԭ�Ӱ뾶��С���� ����Ԫ�ط��ű�ʾ����
(4) д��B��F��J���γɵĻ�����ĵ���ʽ ��
���𰸡�
(1) Ar�� K�� F�� Br��Al�� M (2) H2O��HCl��PH3�� PH3 (3) Cl
(4) 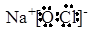 (ÿ��1��)
(ÿ��1��)
������������Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е�λ�ÿɵó�A��F��B��O��C��P��D��C��E��Ar��F��Cl��G��S��H��Al��I��Mg��J��Na��K��Ca��L��K��M��Tc��N��Br��

��ϰ��ϵ�д�
�����Ŀ