��Ŀ����
������У��ø��ܵ��Ӻ��һ���������ʱ�������е�һ�����ӿ��Ա������ȥ���������������ɻ����籽������ӱ������
![]()
�������ӵ�ʽ�������������֮�ȣ�![]() ��Ϊ122����
��Ϊ122����![]() �������ͬʱ�����������ӻ��ᱻ�����������ʽ����Ƭ���ӣ���ֻ��һ����λ������ɣ����������ǵ�
�������ͬʱ�����������ӻ��ᱻ�����������ʽ����Ƭ���ӣ���ֻ��һ����λ������ɣ����������ǵ�![]() ���ɱ������γɵ���Ƭ���Ӱ�����õ�
���ɱ������γɵ���Ƭ���Ӱ�����õ�![]() ��С�����У�122��105��77��51������
��������122��105��77��51������
������ѧ���õ��л�������A������C��H��OԪ����ɣ�ͨ��A��һ����ɫ����Һ�壬������ˮ�����Ⱥ���������ϡNaOH��Һ��ϡH2SO4��Һ����ȴ��������ԭ�е�Һ�塣���ø��ܵ������������A����ʱ����70eV�¿ɵ�![]() Ϊ88��73��61��45��29��27��15�ȵ����ӣ���ֻ��һ������ɣ����ش��������⣺
Ϊ88��73��61��45��29��27��15�ȵ����ӣ���ֻ��һ������ɣ����ش��������⣺
��1���л���A����Է��������� ������ʽ�� ��
��2��![]() Ϊ88�������� ��
Ϊ88�������� ��
��3��![]() Ϊ15�������� ��
Ϊ15�������� ��
����1��88��C4H8O2 ��2��C4H8O2+ ��3��CH3+
����:
����֪A������ʽ����������ɱȵ����ֵ��Ϊ�仯ѧʽ��ʽ�����־�����������Ϣ�����������֪������������A��ʽ��Ϊ88����A�ķ���ʽ��C4H8O2��ͬ�����������Ϣ����֪�����ӡ��ֱ�ΪC4H8O2+��CH3+��

������У��ø��ܵ��Ӻ��һ���������ʱ�������е�һ�����ӿ��Ա������ȥ���������������ɻ����籽������ӱ������
C6H5COOH?? C6H5COOH++e
�������ӵ�ʽ�������������֮�ȣ�![]() ��Ϊ122����
��Ϊ122����![]() �������ͬʱ�����������ӻ��ᱻ�����������ʽ����Ƭ���ӣ���ֻ��һ����λ������ɣ����������ǵ�
�������ͬʱ�����������ӻ��ᱻ�����������ʽ����Ƭ���ӣ���ֻ��һ����λ������ɣ����������ǵ�![]() ���ɱ������γɵ���Ƭ���Ӱ�����õ�
���ɱ������γɵ���Ƭ���Ӱ�����õ�![]() ��С�����У�122��105��77��51������
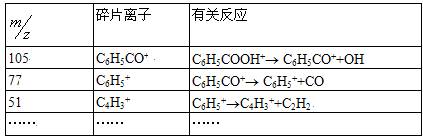
��������122��105��77��51������
|
| ��Ƭ���� | �йط�Ӧ |
| 105 | C6H5CO+ | C6H5COOH+?? C6H5CO++OH |
| 77 | C6H5+ | C6H5CO+?? C6H5++CO |
| 51 | C4H3+ | C6H5+??C4H3++C2H2 |
| ���� | ���� | ���� |
������ѧ���õ��л�������A������C��H��OԪ����ɣ�ͨ��A��һ����ɫ����Һ�壬������ˮ�����Ⱥ���������ϡNaOH��Һ��ϡH2SO4��Һ����ȴ��������ԭ�е�Һ�塣���ø��ܵ������������A����ʱ����70eV�¿ɵ�![]() Ϊ88��73��61��45��29��27��15�ȵ����ӣ���ֻ��һ������ɣ����ش��������⣺
Ϊ88��73��61��45��29��27��15�ȵ����ӣ���ֻ��һ������ɣ����ش��������⣺
��1���л���A����Է��������� ������ʽ�� ��
��2��![]() Ϊ88�������� ��
Ϊ88�������� ��
��3��![]() Ϊ15�������� ��
Ϊ15�������� ��