��Ŀ����
ijУ��ѧ��ȤС��Կα�ʵ����ۡ�������������������ͭ�������ɺ�ɫ��Cu2O������������ɣ���Ϊ��ɫ������һ������������ͭ��Ϊ��ȷ����ɫ�����ijɷ֣���չ�����̽����[һ]������룬������ɲ���2��3��
����1����ɫ����������Cu��
����2��
����3��
[��]�������ϣ���Cu20���ڼ����������Cu+����I���������ܷ�������������ԭ��Ӧ
[��]�Ʊ���ɫ���������Ʊ�������ͭ����Һ��������ͭ����Һ�������ǹ��Ȣ۹��ˡ�ϴ�ӡ����º�ɵú�ɫ��ĩ
[��]��ɫ�����ɷ�̽������С��ͬѧ��������ַ�����
������ȡ�ú�ɫ��ĩ��������ϡ�����У��۲���Һ��ɫ�仯��
������ȡ�ú�ɫ��ĩ��������ϡ�����У��۲��Ƿ��в�����
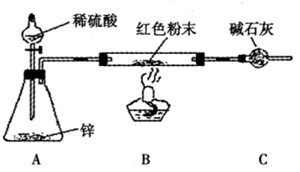
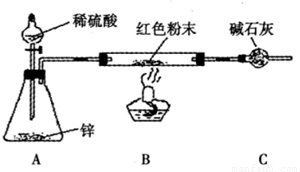
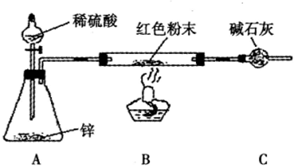
������װ����ͼ��ʾ�����г�������ȥ��
�Իش��������⣺
��1����ֱ����۷���I�ͷ������Ƿ��������ǡ������������ط�Ӧ����ʽ��
����I��
������
��2��ʵ���ϣ��������װ���д������Ե�ȱ�ݣ�Ӧ��A��B֮������
��3���������У�̽��С���ͬѧ����ƿ�м�����������ͭ��Һ����Ŀ����
��4���������У���Ҫ�ⶨ��ɫ��ĩ�ijɷ֣���Ҫ�ⶨ������Щ������
�ٷ�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ������� ��ʵ��ǰ����ܵ����� ��ʵ������ܵ����� ��Ӳ�ʲ����ܵ����� ��п������ ��ϡ��������ʵ���Ũ�ȣ�
������[һ]������룺Cu��Cu2O��Ϊ��ɫ������Ϊ�����Ҳ����Ϊ���ߵĻ���
[��]��ɫ�����ɷ�̽����
��1����Ϊ������Cu2O��Cu�������ﶼ�ɱ������ܽ⣬������ͬ�����������Ҳ����������ΪCu2O��Cu����������������ϡ�����У����в�����
��2���������ԭ�������÷�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ���������ȥӲ�ʲ����ܵ��������ɫ��ĩ��������ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������m��H2O��=0����Cu2O������ɫ��ĩ������=Cu2O����������Cu������ɫ��ĩ���������� Cu2O������������Cu2O��Cu�Ļ�����˷�����Ӧ��A��B֮��Ӹ���װ�ã������������е�ˮ����ֹ��ɸ��ţ�
��3���������У�����ƿ�м�����������ͭ��Һ����Ŀ�����γ�ͭпԭ��أ��ӿ췴Ӧ��
��4�����ݣ�2�����з�����
[��]��ɫ�����ɷ�̽����
��1����Ϊ������Cu2O��Cu�������ﶼ�ɱ������ܽ⣬������ͬ�����������Ҳ����������ΪCu2O��Cu����������������ϡ�����У����в�����
��2���������ԭ�������÷�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ���������ȥӲ�ʲ����ܵ��������ɫ��ĩ��������ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������m��H2O��=0����Cu2O������ɫ��ĩ������=Cu2O����������Cu������ɫ��ĩ���������� Cu2O������������Cu2O��Cu�Ļ�����˷�����Ӧ��A��B֮��Ӹ���װ�ã������������е�ˮ����ֹ��ɸ��ţ�
��3���������У�����ƿ�м�����������ͭ��Һ����Ŀ�����γ�ͭпԭ��أ��ӿ췴Ӧ��
��4�����ݣ�2�����з�����
����⣺[һ]������룺Cu��Cu2O��Ϊ��ɫ������Ϊ�����Ҳ����Ϊ���ߵĻ�������1��ɫ����������Cu������2��ɫ����������Cu2O������3��ɫ����������Cu2O��Cu�Ļ���
�ʴ�Ϊ����ɫ����������Cu2O����ɫ����������Cu2O��Cu�Ļ���
[��]��ɫ�����ɷ�̽����
��1������I��������Cu2O��Cu�������ﶼ�ɱ������ܽ⣬������ͬ��������ϡ�����ܽ⣬Cu20��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ3Cu2O+14HNO3=6Cu��NO3��2+2NO��+7H2O��
�ʴ�Ϊ����3Cu2O+14HNO3=6Cu��NO3��2+2NO��+7H2O��
������Cu2O��Cu����������������ϡ�����У����в�����Ҳ����������Ӧ�����ӷ���ʽΪCu2O+2H+=Cu+Cu2++H2O���ʴ�Ϊ����Cu2O+2H+=Cu+Cu2++H2O��
��2������������Ӧ����ˮ����ͨ��ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������˷�����Ӧ��A��B֮��Ӹ���װ�ã������������е�ˮ����ֹ��ɸ��ţ�
�ʴ�Ϊ������װ�ã�
��3���������У�����ƿ�м�����������ͭ��Һ����Ŀ�����γ�ͭпԭ��أ��ӿ췴Ӧ���ʴ�Ϊ���γ�ͭпԭ��أ��ӿ췴Ӧ��
��4���������ԭ�������÷�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ���������ȥӲ�ʲ����ܵ��������ɫ��ĩ��������ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������m��H2O��=0����Cu2O������ɫ��ĩ������=Cu2O����������Cu������ɫ��ĩ���������� Cu2O������������Cu2O��Cu�Ļ�������Ҫ�����ٷ�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ������� ��ʵ��ǰ����ܵ����� ��ʵ������ܵ����� ��Ӳ�ʲ����ܵ�������
�ʴ�Ϊ���٢ڢۢܣ�
�ʴ�Ϊ����ɫ����������Cu2O����ɫ����������Cu2O��Cu�Ļ���
[��]��ɫ�����ɷ�̽����
��1������I��������Cu2O��Cu�������ﶼ�ɱ������ܽ⣬������ͬ��������ϡ�����ܽ⣬Cu20��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ3Cu2O+14HNO3=6Cu��NO3��2+2NO��+7H2O��
�ʴ�Ϊ����3Cu2O+14HNO3=6Cu��NO3��2+2NO��+7H2O��
������Cu2O��Cu����������������ϡ�����У����в�����Ҳ����������Ӧ�����ӷ���ʽΪCu2O+2H+=Cu+Cu2++H2O���ʴ�Ϊ����Cu2O+2H+=Cu+Cu2++H2O��
��2������������Ӧ����ˮ����ͨ��ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������˷�����Ӧ��A��B֮��Ӹ���װ�ã������������е�ˮ����ֹ��ɸ��ţ�
�ʴ�Ϊ������װ�ã�
��3���������У�����ƿ�м�����������ͭ��Һ����Ŀ�����γ�ͭпԭ��أ��ӿ췴Ӧ���ʴ�Ϊ���γ�ͭпԭ��أ��ӿ췴Ӧ��
��4���������ԭ�������÷�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ���������ȥӲ�ʲ����ܵ��������ɫ��ĩ��������ʵ��ǰ�����ܵ�������ˮ���������Ӷ���Cu2O����������m��H2O��=0����Cu2O������ɫ��ĩ������=Cu2O����������Cu������ɫ��ĩ���������� Cu2O������������Cu2O��Cu�Ļ�������Ҫ�����ٷ�Ӧǰ��ɫ��ĩ��Ӳ�ʲ����ܵ������� ��ʵ��ǰ����ܵ����� ��ʵ������ܵ����� ��Ӳ�ʲ����ܵ�������
�ʴ�Ϊ���٢ڢۢܣ�
���������⿼�黯ѧʵ���̽������ơ����ۡ����ݴ�����֪ʶ����ѧ��������Ҫ��ϸߣ�������ѧ���ķ���������ʵ�������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬ע��������ʵ������Լ�ʵ��ԭ����˼�����Ѷ��еȣ�

��ϰ��ϵ�д�
 �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ