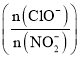��Ŀ����
����Ŀ��ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.00mL����ƿ�У�����10.00mLKI��Һ��������������ָʾ��2��3�Ρ�
��ȡһֻ��ʽ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0.010mol��L��1Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2��2Na2S2O3=2NaI��Na2S4O6��
�Իش��������⣺
��1������ټ����ָʾ����___��
��2���ζ�ʱ���۾�Ӧע��___���жϵ���ζ��յ��������__������ȥNa2S2O3��Һ20.00mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ___��
��3��ʵ���У�Cl2������Ũ�ȱ�ʵ��Ũ��ƫ���������ԭ����___��
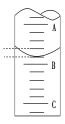
��4����ͼ��ʾ50.00mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25.00mL���ζ�����Һ�����ӦΪ___mL�����ʱҺ���������ΪamL���ζ�����Һ������V___(50��a)mL������<��������������>������
��5���ζ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNa2S2O3��Һ��������ᵼ�²ⶨ���__������ƫ��������ƫС��������Ӱ��������
���𰸡�������Һ ��ƿ����Һ����ɫ�仯 ��ƿ����Һ����ɫ����ɫ��dz����ɫ�ұ���30���ڲ��ָ� 0.01mol��L-1 ��Ϊ����ڵζ���������ˮϴ����δ�ô���Һ��ϴ���ʲ����c(Cl2)����ʵ��Ũ�� 25.40 > ƫС
��������
������̬����⻯�ط�Ӧ���ɵ��ʵ⣬���ʵ�����������Ʒ�Ӧ�������ʵ����ģ�����Ҫ�õ�����Һ����ָʾ�����ʴ�Ϊ������Һ��
�Ƶζ�ʱ���۾�Ӧע����ƿ����Һ����ɫ�仯���жϵ���ζ��յ����������ƿ����Һ����ɫ����ɫ��Ϊ��ɫ�ұ���30���ڲ��ָ���
����ȥNa2S2O3��Һ20.00mL�����ˮ��Cl2�����ʵ���Ũ��Ϊ
I2��2Na2S2O3=2NaI��Na2S4O6�� Cl2 �� 2I�� == 2 Cl����I2

![]()
x =1 ��10��4 mol
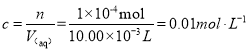
��ʵ���У�Cl2������Ũ�ȱ�ʵ��Ũ��ƫ���������ԭ������Ϊ�������ζ���������ˮϴ����δ�ô���Һ��ϴ����˲����c(Cl2)����ʵ��Ũ�ȣ��������ǿ�ʼ�����ݣ������ⶨ���û�����ݵ�������ʴ�Ϊ��Ϊ�������ζ���������ˮϴ����δ�ô���Һ��ϴ���ʲ����c(Cl2)����ʵ��Ũ�ȣ�
����ͼ��ʾ50.00mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25.00mL���ζ���ÿһ���̶�Ϊ0.10mL���ζ���Ҫ��ȷ��0.01mL�����Һ�����ӦΪ25.40mL�����ʱҺ���������ΪamL���������̶��·����кܶ�Һ���ڵζ����У�����Һ����������(50��a)mL���ʴ�Ϊ25.40mL��>��
�ɵζ�����ʱ���ӿ̶��߶�ȡ�ζ��յ㣬�����ڵζ�������̶�С�����ж���������ƫС�����ƫС�����ƫС���ʴ�ΪƫС��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�