��Ŀ����
14�����ͼ��ѡ�ñ�Ҫ��װ�ý��е�ⱥ��ʳ��ˮ��ʵ�飬Ҫ��ⶨ�������������������25mL���������������������ԣ�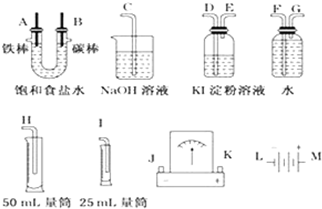
��1��A�������ĵ缫��Ӧʽ�ǣ�2H++2e-�TH2����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2����
��2����Դ����������A��B��������ȷ����˳��Ϊ��L��A��B��J��K��M��
��3�������������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ��H��F��G��A��B��D��E��C��
��4����֪����ʳ��ˮ50mL��ijʱ�̲��H2���Ϊ5.6mL����״��������ʱ��ҺpHԼΪ12��
���� ��1��Ҫ��������̼����������Ȼ�����Һ��ȡ������������������̼���������������������ӷŵ����������������������ӷŵ�����������
��2����Դ�����ӵ��ص�������̼���ӵ����ơ�-���ˣ���+���˽ӵ�Դ������
��3���õ⻯����Һ���������������ԣ�������������Һ����������β��������ˮ���ռ���������50mL��Ͳʢ���ų���ˮ��ע�������ѭ�������̳���ԭ��
��4�������������������ƵĹ�ϵ�Ǽ����������Ƶ����ʵ������ٸ���c=$\frac{n}{V}$������������Ũ�ȣ��ٽ�����ӻ���������������Ũ�ȣ��Ӷ��ó���Һ��pH��
��� �⣺��1��Ҫ��������̼����������Ȼ�����Һ��ȡ������������������̼�����������������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-�TH2��������̼����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ2Cl--2e-�TCl2����ͬʱ��Һ�л������������ƣ����Ե�ط�ӦʽΪ 2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2����
�ʴ�Ϊ��2H++2e-�TH2����2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2����
��2����Դ�����ӵ��ص�������̼���ӵ����ơ�-���ˣ���+���˽ӵ�Դ����������������˳����A��B��J��K��
�ʴ�Ϊ��A��B��J��K��
��3���������A���ܿڲ���H2���ұ�B���ܿڲ���Cl2���Ե���Ϊ���ģ���Ӧװ�õ����ã� ����������˳���ǣ�H��F��G��A��B��D��E��C��
����������˳���ǣ�H��F��G��A��B��D��E��C��
�ʴ�Ϊ��H��F��G��D��E��C��
��4������2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2�����������ƺ������Ĺ�ϵʽ֪��n��NaOH��=$\frac{\frac{5.6��1{0}^{-3}L}{22.4L/mol}}{1}$=5.0��10-4 mol��
����ԭ���غ��n��OH-��=5.0��10-4 mol��c��OH-��=��5.0��10-4 mol���£�50��10-3 L��=10-2 mol/L��c��H+��=$\frac{1{0}^{-14}}{c��O{H}^{-}��}$=$\frac{1{0}^{-14}}{0.02}$=10-12 mol/L��pH=-lg10-12=12��
�ʴ�Ϊ��12��
���� �����Ե��ԭ��Ϊ���忼�����������ȡ�����ʵļ����֪ʶ�㣬���ݵ��ԭ�������ʵ����ʡ����ʼ�Ĺ�ϵ����������ɣ��ѵ�����������˳����������ȡװ�á�����װ�á��ռ�װ�á�β������װ�������ɣ��Ѷ��еȣ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�| A�� | pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ��c��NaOH����c��CH3COONa����c��Na2CO3�� | |
| B�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��CH3COOH�� | |
| C�� | 0.1 mol•L-1 ��NaHA��Һ����pH=4��c��HA-����c��H+����c��H2A����c��A2-�� | |
| D�� | 25��ʱpH=2��HA��Һ��pH=12��MOH��Һ����Ȼ�ϣ�c��H+��+c��M+��=c��OH-��+c��A-�� |
| A�� | �Ƚ�Cu��Fe�Ļ�ԭ�ԣ�ͭ������������Һ�� | |
| B�� | �Ƚ�þ�����Ľ����ԣ�ȡһС��ȥ����Ĥ��þ������Ƭ���ֱ����1.0 mol•L-1�������� | |
| C�� | �Ƚϸ�����ء������������ԣ���������м���Ũ���� | |
| D�� | �Ƚ��ȡ���ķǽ����ԣ��廯����Һ��ͨ������ |
| A�� | 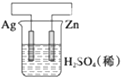 ������ӦʽΪ2H++2e-�TH2�� | B�� | 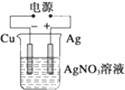 ������ӦʽΪAg++e-�TAg | ||
| C�� | 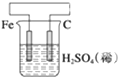 H+��̼�������ƶ� | D�� | 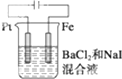 ��ʼʱ������������ɫ���� |
| A�� | ��״���£�22.4 L��ˮ����NA��Cl2���� | |
| B�� | ��1 L 0.1 mol•L-1̼������Һ����������������0.1NA | |
| C�� | 50 mL18.4 mol•L-1Ũ����������ͭ�ȷ�Ӧ������SO2������ĿΪ0.46NA | |
| D�� | ��״���£���H2O2�Ƶ�4.48 LO2ת�Ƶĵ�����ĿΪ0.8NA |
| A�� | ������ˮ������ǿ����ʣ�������ˮ�����Ƿǵ���� | |
| B�� | ǿ�������Һ�в����ڷ��ӣ����������Һ�бش������ʷ��� | |
| C�� | ������״̬���ܵ���Ļ�����һ�������ӻ����Ҳһ����ǿ����� | |
| D�� | ǿ�����һ���ܵ��磬�ǵ���ʵ�ˮ��Һһ�������� |


 Al3++3HCO3-=Al��OH��3��+3CO2����
Al3++3HCO3-=Al��OH��3��+3CO2���� Al��OH��3+3H+�TAl3++3H2O��
Al��OH��3+3H+�TAl3++3H2O��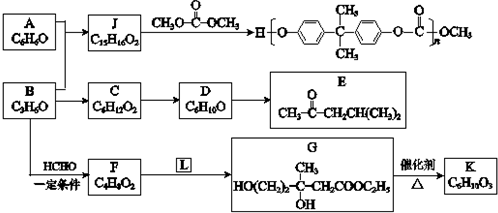
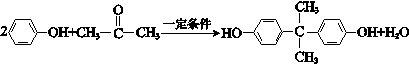 ����
����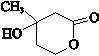 ��
��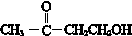 ��
��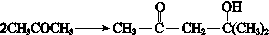 ��
��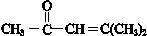 ��
��