��Ŀ����
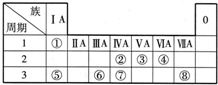 ��ͼ��ʾΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
��ͼ��ʾΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺��1���ܢݢ�ԭ�Ӱ뾶�ɴ�С��˳����
Na��Al��O
Na��Al��O
����Ԫ����ţ�����2���ڢۢߵ���ۺ������������ǿ������˳����
HNO3��H2CO3��H2SiO3
HNO3��H2CO3��H2SiO3
���ѧʽ������3���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ
ab
ab
������ţ���a��MnO2
b��FeCl3
c��Na2SO3
d��KMnO4
��4���ɱ���Ԫ����ɵij�������x��Y��Z��M��N�ɷ������·�Ӧ��
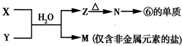
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ
Al3++3NH3?H2O=Al��OH��3��+3NH4+
Al3++3NH3?H2O=Al��OH��3��+3NH4+
��N���ĵ��ʵĻ�ѧ����ʽΪ
2Al2O3�����ڣ�
4Al+3O2��
| ||
2Al2O3�����ڣ�
4Al+3O2��
��
| ||
�����£�Ϊʹ0.1mol/L M��Һ����M���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ��
����
����
����������Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͬ�����������ԭ�Ӱ뾶��С��
��2���ǽ�����Խǿ��ۺ����������Խǿ��
��3��1��1��ɵij���Һ̬������ΪH2O2��MnO2��FeCl3��H2O2�ֽ�Ĵ�����
��4����ΪAl�����Ƶ�NΪAl2O3��ZΪAl��OH��3��M�ǽ����ǽ������Σ�����Mһ������Σ��ɴ��Ƴ�X��Y�ķ�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ������Ϊ�Ȼ���������������M����Ϊ�Ȼ�炙�����泥�笠�����ˮ�⣬ʹ0.1mol/L M��Һ����M���������������Ũ����ȣ����ݵ���غ��֪c��OH-��=c��H+����
��1�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͬ�����������ԭ�Ӱ뾶��С��
��2���ǽ�����Խǿ��ۺ����������Խǿ��
��3��1��1��ɵij���Һ̬������ΪH2O2��MnO2��FeCl3��H2O2�ֽ�Ĵ�����
��4����ΪAl�����Ƶ�NΪAl2O3��ZΪAl��OH��3��M�ǽ����ǽ������Σ�����Mһ������Σ��ɴ��Ƴ�X��Y�ķ�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ������Ϊ�Ȼ���������������M����Ϊ�Ȼ�炙�����泥�笠�����ˮ�⣬ʹ0.1mol/L M��Һ����M���������������Ũ����ȣ����ݵ���غ��֪c��OH-��=c��H+����
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͬ�����������ԭ�Ӱ뾶��С������ԭ�Ӱ뾶Na��Al��O���ʴ�Ϊ��Na��Al��O��
��2��ͬ�������϶��·ǽ����Լ�����ͬ����������ҷǽ�������ǿ���ʷǽ�����N��C��Si���ǽ�����Խǿ��ۺ����������Խǿ��������HNO3��H2CO3��H2SiO3��
�ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3��1��1��ɵij���Һ̬������ΪH2O2��MnO2��FeCl3��H2O2�ֽ�Ĵ�������ѡab��
��4����ΪAl�����Ƶ�NΪAl2O3��ZΪAl��OH��3��M�ǽ����ǽ������Σ�����Mһ������Σ��ɴ��Ƴ�X��Y�ķ�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ������Ϊ�Ȼ���������������M����Ϊ�Ȼ�炙�����泥�
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
N���ĵ��ʵĻ�ѧ����ʽΪ��2Al2O3�����ڣ�
4Al+3O2����
笠�����ˮ�⣬ʹ0.1mol/L M��Һ����M���������������Ũ����ȣ����ݵ���غ��֪c��OH-��=c��H+��������Һ�����ԣ�
�ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��2Al2O3�����ڣ�
4Al+3O2�������ԣ�
��1�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͬ�����������ԭ�Ӱ뾶��С������ԭ�Ӱ뾶Na��Al��O���ʴ�Ϊ��Na��Al��O��
��2��ͬ�������϶��·ǽ����Լ�����ͬ����������ҷǽ�������ǿ���ʷǽ�����N��C��Si���ǽ�����Խǿ��ۺ����������Խǿ��������HNO3��H2CO3��H2SiO3��
�ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3��1��1��ɵij���Һ̬������ΪH2O2��MnO2��FeCl3��H2O2�ֽ�Ĵ�������ѡab��
��4����ΪAl�����Ƶ�NΪAl2O3��ZΪAl��OH��3��M�ǽ����ǽ������Σ�����Mһ������Σ��ɴ��Ƴ�X��Y�ķ�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ������Ϊ�Ȼ���������������M����Ϊ�Ȼ�炙�����泥�
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
N���ĵ��ʵĻ�ѧ����ʽΪ��2Al2O3�����ڣ�
| ||
笠�����ˮ�⣬ʹ0.1mol/L M��Һ����M���������������Ũ����ȣ����ݵ���غ��֪c��OH-��=c��H+��������Һ�����ԣ�
�ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��2Al2O3�����ڣ�
| ||
���������⿼��������ƶ��Լ�Ԫ�����ڱ��������ɵ��ۺ�Ӧ�ã���Ŀ����ǶȽ϶࣬�Ѷ��еȣ�ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
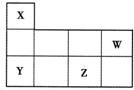
��ͼ��ʾΪԪ�����ڱ���һ���֣�X��Y��W��Z��Ϊ������Ԫ�أ�����ֻ��XΪ����Ԫ�ء�����˵���������
|
|
W |
Z |
|
X |
Y |
|
A��ԭ�Ӱ뾶��Z��W��Y��X
B��Ԫ��Y������������NaOH��Һ��Ӧ
C�������̬�⻯������ȶ��ԣ�Y��W
D��W��Z�������ﶼ����Ӧ�����κ�ˮ
��ͼ��ʾΪԪ�����ڱ���һ���֣�X��Y��W��Z��Ϊ������Ԫ�أ�����ֻ��XΪ����Ԫ�ء�����˵���������
|
|
W |
Z |
|
X |
Y |
|
A��ԭ�Ӱ뾶��Z��W��Y��X
B��Ԫ��Y������������NaOH��Һ��Ӧ
C�������̬�⻯������ȶ��ԣ�Y��X
D��W��Z�������ﶼ����Ӧ�����κ�ˮ
 ��ͼ��ʾΪԪ�����ڱ������ڵ�һ���֣����й���Y��Z��W��˵������ȷ���ǣ�������
��ͼ��ʾΪԪ�����ڱ������ڵ�һ���֣����й���Y��Z��W��˵������ȷ���ǣ������� �ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺
�ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺