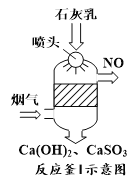
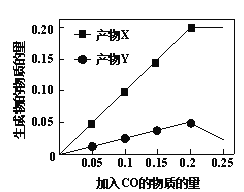
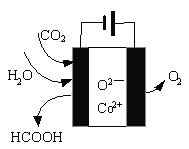
��Ŀ����
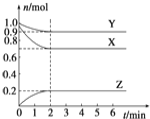
����Ŀ��ijͬѧ��ȡһ��������MgCl2���������Һ���ڸ���Һ�м���һ������ϡ���ᣬȻ����˻����Һ����μ���NaOH��Һ(��ͼ����ʾ)���μӹ����в������������������NaOH��Һ������Ĺ�ϵ��ͼ����ʾ��

��ش��������⣺
(1)�ܽ�MgCl2�������õIJ���������________(����ĸ)��
a.��ƽ������b.�ձ�������c.©��������d.������
(2)OA�η�Ӧ�����ӷ���ʽΪ____________________________________________________________
(3)AB�η�Ӧ�����ӷ���ʽΪ____________________________________________________________
���𰸡�bd H+ + OH�� = H2O Mg2+ + 2OH�� = Mg(OH)2![]()
��������
�����Һ�м����������ƣ����������������ᷴӦ�������Ȼ�þ��Ӧ����������þ������
(1)�ܽ�MgCl2�������õIJ����������ձ��Ͳ�������
(2)OA�η�ӦΪ������������Ʒ�Ӧ�����ӷ���ʽΪ��H+ + OH�� = H2O��
(3)AB�η�ӦΪ�Ȼ�þ���������Ʒ�Ӧ����������þ���������ӷ���ʽΪMg2+ + 2OH�� = Mg(OH)2![]() ��
��

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ