ЬтФПФкШн
НЋвЛЖЈСПЕФAg2SO4ЙЬЬхжУгкШнЛ§ВЛБфЕФШнЦїжаЃЌдкФГЮТЖШЯТЗЂЩњЯТСаЗДгІЃК
Ag2SO4(s) Ag2O(s)+SO3(g) 2SO3(g)
Ag2O(s)+SO3(g) 2SO3(g) 2SO2(g)+O2(g)
2SO2(g)+O2(g)
О10ЗжжгЗДгІДяЕНЦНКтЃЌДЫЪБc(SO3)=0.4 mol/LЃЌc(O2)=0.05 mol/LЃЌЯТСаа№ЪіВЛе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎSO3ЕФЗжНтТЪЮЊ20% BЃЎ10ЗжжгФкІЭ(SO2)=0.01mol/(LЁЄmin)
CЃЎШнЦїФкЦјЬхЕФУмЖШЮЊ40g/L DЃЎМгбЙЃЌШнЦїФкЙЬЬхЕФжЪСПВЛБф
D

дквЛЬхЛ§ПЩБфЕФУмБеШнЦїжаЃЌМгШывЛЖЈСПЕФXЁЂYЃЌЗЂЩњЗДгІmX(g) nY(g)ЃЌІЄHЃНQ kJ/molЁЃЗДгІДяЕНЦНКтЪБЃЌYЕФЮяжЪЕФСПХЈЖШгыЮТЖШЁЂШнЦїЬхЛ§ЕФЙиЯЕШчЯТБэЫљЪОЃК
nY(g)ЃЌІЄHЃНQ kJ/molЁЃЗДгІДяЕНЦНКтЪБЃЌYЕФЮяжЪЕФСПХЈЖШгыЮТЖШЁЂШнЦїЬхЛ§ЕФЙиЯЕШчЯТБэЫљЪОЃК
|
| 1 | 2 | 4 |
| 100 | 1.00 | 0.75 | 0.53 |
| 200 | 1.20 | 0.90 | 0.63 |
| 300 | 1.30 | 1.00 | 0.70 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. m>n B. ЮТЖШВЛБфЃЌбЙЧПдіДѓЃЌYЕФжЪСПЗжЪ§МѕЩй
C. Q<0 D. ЬхЛ§ВЛБфЃЌЮТЖШЩ§ИпЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏ
ЂХвбжЊ4NH3(g)+5O2(g)ЃН4NO(g) +6H2O(l)ЃЌЁїHЃНx kJ/molЁЃеєЗЂ1mol вКЬЌЫЎашвЊЮќЪеЕФФмСПЮЊ44kJЃЌЦфЫќЯрЙиЪ§ОнШчЯТБэЃК
| NH3(g) | O2(g) | NO(g) | H2O(g) | |
| 1molЗжзгЖЯСбЛЏбЇМќЪБашвЊЮќЪеЕФФмСП/kJ | a | b | z | d |
дђБэжаzЃЈгУxЁЂaЁЂbЁЂdБэЪОЃЉЕФДѓаЁЮЊ______________________________________________
ЂЦвбжЊa g ввЯЉЦјЬхГфЗжШМЩеЪБЩњГЩ1molCO2КЭвКЬЌЫЎЃЌЗХГіb k JЕФШШСПЃЌдђввЯЉШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ________________________________________________ЁЃ
ЂЧРћгУЗДгІ2CuЃЋO2ЃЋ2H2SO4ЃН2CuSO4ЃЋ2H2OПЩвджЦБИCuSO4ЃЌШєНЋИУЗДгІЩшМЦЮЊдЕчГиЃЌЦфе§МЋЕчМЋЗДгІЪНЮЊ ЁЃ
ЂШыТ(N2H4)ЁЊПеЦјШМСЯЕчГиЪЧвЛжжМюадШМСЯЕчГиЃЌЕчНтжЪШмвКЪЧ20%~30%ЕФKOHШмвКЁЃИУЕчГиЗХЕчЪБЃЌИКМЋЕФЕчМЋЗДгІЪНЪЧ_______________________________ЁЃ
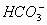 )ЁЂc(
)ЁЂc(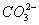 )ЖММѕЩйЃЌЦфЗНЗЈЪЧ ЃЈ ЃЉ
)ЖММѕЩйЃЌЦфЗНЗЈЪЧ ЃЈ ЃЉ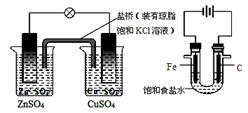
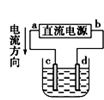
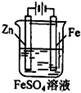
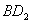 ЗжзгЕФЕчзгЪНЮЊ__________ЃЌ
ЗжзгЕФЕчзгЪНЮЊ__________ЃЌ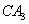 ЗжзгЕФПеМфСЂЬхЙЙаЭЮЊ__________ЁЃ
ЗжзгЕФПеМфСЂЬхЙЙаЭЮЊ__________ЁЃ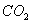 КЭвКЬЌ
КЭвКЬЌ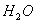 ЃЌЗХГіQ kJШШСПЃЌдђБэЪОHЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ____________________ЁЃФГШМСЯЕчГигУHзїШМСЯЃЌKOHЮЊЕчНтвКЃЌИУЕчГиЕФИКМЋЕчМЋЗДгІЪНЮЊЃК________________________________________ЁЃ
ЃЌЗХГіQ kJШШСПЃЌдђБэЪОHЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ____________________ЁЃФГШМСЯЕчГигУHзїШМСЯЃЌKOHЮЊЕчНтвКЃЌИУЕчГиЕФИКМЋЕчМЋЗДгІЪНЮЊЃК________________________________________ЁЃ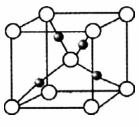
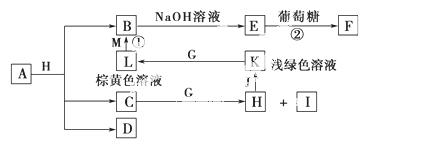
 зюЭтВуЕчзгЪ§жЎКЭЮЊ10ЁЃDЮЊЮоЩЋЦјЬхЧвВЛФмШМЩеЃЌGЮЊЛЦТЬЩЋЕЅжЪЦјЬхЃЌJЁЂMЮЊН№ЪєЃЌ
зюЭтВуЕчзгЪ§жЎКЭЮЊ10ЁЃDЮЊЮоЩЋЦјЬхЧвВЛФмШМЩеЃЌGЮЊЛЦТЬЩЋЕЅжЪЦјЬхЃЌJЁЂMЮЊН№ЪєЃЌ IгаЦЏАззїгУЃЌЗДгІЂйГЃгУгкжЦзїгЁЫЂЕчТЗАхЁЃЧыЛиД№
IгаЦЏАззїгУЃЌЗДгІЂйГЃгУгкжЦзїгЁЫЂЕчТЗАхЁЃЧыЛиД№