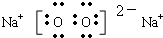��Ŀ����
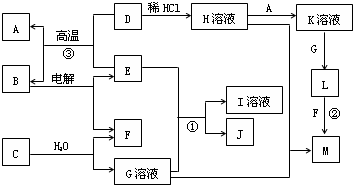
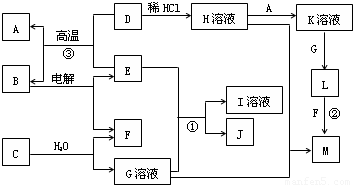
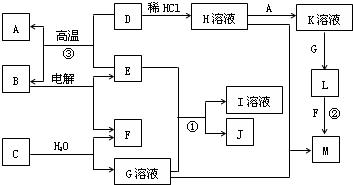
���¿�ͼ��A��M����ѧ��ѧ���������ʣ�����A��E�ǽ�����F��JΪ���嵥�ʣ������Ϊ�����������Һ��CΪ����ɫ���壬DΪ����ɫ��ĩ��MΪ���ɫ���壮
��ش��������⣺
��1��B�Ļ�ѧʽ______��
��2��19.5gC��������ˮ��Ӧת�Ƶ��ӵ����ʵ���Ϊ______mol��
��3������H��Һ�������ɡ����գ����յõ��Ĺ������ʵĻ�ѧʽ______��
��4��д���ٷ�Ӧ�����ӷ���ʽ______��
��5��д���ڡ��۷�Ӧ�Ļ�ѧ����ʽ��______����______��

��ش��������⣺
��1��B�Ļ�ѧʽ______��
��2��19.5gC��������ˮ��Ӧת�Ƶ��ӵ����ʵ���Ϊ______mol��
��3������H��Һ�������ɡ����գ����յõ��Ĺ������ʵĻ�ѧʽ______��
��4��д���ٷ�Ӧ�����ӷ���ʽ______��
��5��д���ڡ��۷�Ӧ�Ļ�ѧ����ʽ��______����______��
CΪ����ɫ���壬����ˮ��Ӧ����G��Һ��F���嵥�ʣ���CΪNa2O2��GΪNaOH��FΪO2��
DΪ����ɫ��ĩ��ΪFe2O3��MΪ���ɫ���壬ΪFe��OH��3����D
H��Һ��H��Һ
M��֪��HΪFeCl3����H
K��Һ
L
Fe��OH��3����LΪFe��OH��2��A�ǽ�������AΪFe��KΪFeCl2��
�ɷ�Ӧ��Fe2O3+����E
Fe+B���������ȷ�Ӧ��EΪAl��BΪAl2O3�����Al2O3����Al��O2������ת����ϵ���ɷ�Ӧ��Al��NaOH��Һ��Ӧ����NaAlO2��H2��JΪ���嵥�ʣ���JΪH2��IΪNaAlO2��
��1��������������֪��BΪAl2O3��
�ʴ�Ϊ��Al2O3��
��2��CΪNa2O2����ˮ��ӦΪ2Na2O2+2H2O=4NaOH+O2������Ӧ��OԪ�ػ��ϼ���-1�۽���Ϊ-2�ۣ���-1����Ϊ0�ۣ�Na2O2�������������ǻ�ԭ������ռ
��19.5gNa2O2�����ʵ���Ϊ
=0.25mol��Na2O2��ȫ��Ӧת�Ƶ������ʵ���Ϊ0.25mol��
��2=0.25mol��
�ʴ�Ϊ��0.25��
��3��FeCl3��Һ�д���ƽ��FeCl3+3H2O?Fe��OH��3+3HCl���������ɣ�HCl�ӷ����ٽ�ˮ�⳹�ף�����Fe��OH��3������Fe��OH��3�ֽ�����Fe2O3��ˮ�������յõ��Ĺ��������ǣ�Fe2O3��
�ʴ�Ϊ��Fe2O3��
��4����Ӧ����Al��NaOH��Һ��Ӧ����NaAlO2��H2����Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=AlO2-+3H2����
��5����Ӧ����Fe��OH��2��O2����ΪFe��OH��3����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��Ӧ����Al��Fe2O3�ڸ��������·�Ӧ����Fe��Al2O3����Ӧ����ʽΪ��Fe2O3+2Al
2Fe+Al2O3��
�ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��Fe2O3+2Al
2Fe+Al2O3��
DΪ����ɫ��ĩ��ΪFe2O3��MΪ���ɫ���壬ΪFe��OH��3����D
| ���� |
| NaOH��Һ |
| A |
| NaOH��Һ |
| O2 |
�ɷ�Ӧ��Fe2O3+����E
| ���� |
��1��������������֪��BΪAl2O3��
�ʴ�Ϊ��Al2O3��
��2��CΪNa2O2����ˮ��ӦΪ2Na2O2+2H2O=4NaOH+O2������Ӧ��OԪ�ػ��ϼ���-1�۽���Ϊ-2�ۣ���-1����Ϊ0�ۣ�Na2O2�������������ǻ�ԭ������ռ
| 1 |
| 2 |
| 19.5g |
| 78g/mol |
| 1 |
| 2 |
�ʴ�Ϊ��0.25��
��3��FeCl3��Һ�д���ƽ��FeCl3+3H2O?Fe��OH��3+3HCl���������ɣ�HCl�ӷ����ٽ�ˮ�⳹�ף�����Fe��OH��3������Fe��OH��3�ֽ�����Fe2O3��ˮ�������յõ��Ĺ��������ǣ�Fe2O3��
�ʴ�Ϊ��Fe2O3��
��4����Ӧ����Al��NaOH��Һ��Ӧ����NaAlO2��H2����Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=AlO2-+3H2����
��5����Ӧ����Fe��OH��2��O2����ΪFe��OH��3����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��Ӧ����Al��Fe2O3�ڸ��������·�Ӧ����Fe��Al2O3����Ӧ����ʽΪ��Fe2O3+2Al
| ||
�ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��Fe2O3+2Al
| ||

��ϰ��ϵ�д�
 53������ϵ�д�
53������ϵ�д�
�����Ŀ