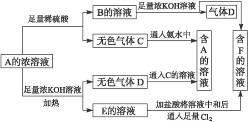
��Ŀ����
��ij����������NH3��H2O��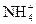 ��H3O+��
��H3O+��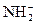 ��OH-��N3-��O2-���ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� ��
��OH-��N3-��O2-���ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� ��
��2Na+2NH3��l��====2NaNH2+H2��
��CaO+2NH4Cl====CaCl2+2NH3��+H2O
��3Mg(NH2)2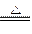 Mg3N2+4NH3��
Mg3N2+4NH3��
��NH4Cl+NaNH2====NaCl+2NH3��
��Mg3N2+4NH3(l)====3Mg(NH2)2
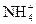 ��H3O+��
��H3O+��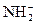 ��OH-��N3-��O2-���ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� ��
��OH-��N3-��O2-���ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� ����2Na+2NH3��l��====2NaNH2+H2��
��CaO+2NH4Cl====CaCl2+2NH3��+H2O
��3Mg(NH2)2
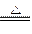 Mg3N2+4NH3��
Mg3N2+4NH3����NH4Cl+NaNH2====NaCl+2NH3��
��Mg3N2+4NH3(l)====3Mg(NH2)2
| A���٢� | B���ۢ� | C��ֻ�Т� | D���٢ڢۢܢ� |
D
����Ϊ��Ӧ���ɵ�Ǩ���⣬�ɰ����⣬��������ʽ�к�������������Ļ����Ȼ���ٰ�����ѧ���ĺ�����������йط�Ӧ�����жϡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ