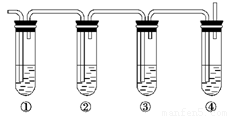
��Ŀ����
ʵ������ȡSO2�ķ�Ӧԭ��Ϊ��Na2SO3+H2SO4(��Ũ)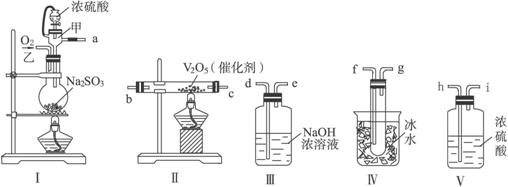
(1)��Щװ�õ�����˳��(���������ҵķ���)��_________��_________��_________�� _________��_________��_________��_________��__________(����ӿڵı��)��
(2)ʵ��ʱ��������������ԭ����_________________________________________________��
(3)���Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳����____________��
(4)�����۲쵽��������_____________________________________________��
(5)��n mol Na2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ��Ƶâ�����m g����ʵ����SO2��ת����Ϊ___________��
�������������ȡ������Ҫ���ǵ����������ӡ���ȡ��Ӧ���ռ���β�����������ԣ�װ�õ�����˳����a h i b c f g d���ڶ�����ѹǿԭ����Ӧ�á��ش�����ʱ��һ���μ�ʵ��Ŀ�ģ��Ͳ��ѻش����⡣
�𰸣�(1)a h i b c f g d
(2)������ʹŨ������˳���ص�����ƿ�У�ԭ����ά����ƿ��ѹǿ���Һ©����ѹǿ���
(3)�ȼ���V2O5��������Ũ����
(4)����ɫ(���ɫ)����(�����)����
(5)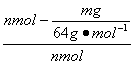 ��100%��
��100%��![]() ��100%
��100%

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�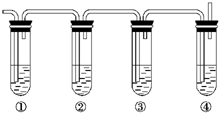 ʵ������ȡ��ϩ�ķ�Ӧԭ��ΪCH3CH2OH
ʵ������ȡ��ϩ�ķ�Ӧԭ��ΪCH3CH2OH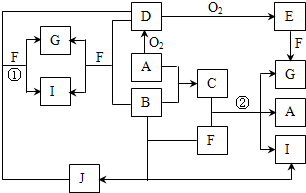 �ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�
�ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
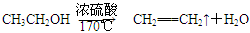 ,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������