��Ŀ����
�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
500 | 800 | ||
��2H2(g)+CO(g) | K1 | 2.5 | 0.15 |
��H2(g)+CO2(g) | K2 | 1.00 | 2.5 |
��3H2(g)+CO2(g) | K3 | ||
(1)�����һ�����ܱ������з�����Ӧ�ڣ��ﵽƽ��������¶ȣ�����˵����ȷ����____________
A.ƽ�������ƶ� B.�ﵽ�µ�ƽ�����ϵ��ѹǿ����
C.H2��ת�������� D.��ϵ���ܶ�����
(2)ij�¶��·�Ӧ����H2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������ʼ����2molH2��1molCO��A��ʱ���������Ϊ1L����B��Ļ�ѧƽ�ⳣ��Ϊ_______��

(3)�ݷ�Ӧ����ڿ��Ƶ���Kl��K2��K3֮��Ĺ�ϵ����K3=__________��(��K1��K2��ʾ)��500��ʱ��÷�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O(g) ��Ũ��(mol/L)�ֱ�Ϊ0.8��0.1��0.3��0.15,���ʱv��_______ v��(�> ������= ����< ")��
(4)��3L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO)һ��Ӧʱ��t�仯����l��ͼ��ʾ������t0ʱ�̷ֱ�ı�һ������������I��Ϊ����II������III��

������I��Ϊ����IIʱ���ı������������________��������I��Ϊ����IIIʱ���ı������������___________��
(5)һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣͨ��״���£���a mol/L�Ĵ�����bmol/LBa (OH)2��Һ�������ϣ���Ӧƽ��ʱ��2c(Ba2+)=c(CH3COO-)���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ_______��
 ��У����ϵ�д�
��У����ϵ�д� CH3OH(g)
CH3OH(g)

 H2 (g)+CO2(g) ��H<0��
H2 (g)+CO2(g) ��H<0��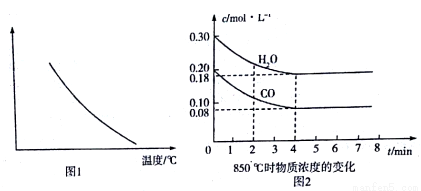
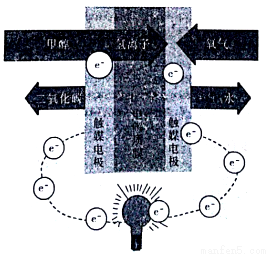
 ���ӽṹ��������������ȷ����
���ӽṹ��������������ȷ����