��Ŀ����
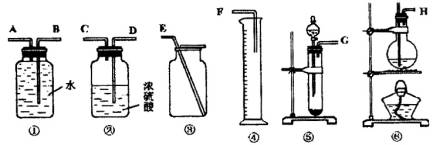
����һ���������Ѻ��Ҵ��Ļ���������������ͼ��ѡ���ʵ���ʵ��װ�ã����һ�����ʵ�飬�ⶨ�������Ҵ��ĺ������ɹ�ѡ�õķ�Ӧ����Լ�Ϊ�����Ƶ���ʯ�ҡ�ŨH2SO4������ˮ�������ơ���ʯ�ҡ���ˮ����ͭ��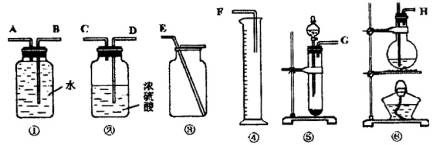
(1)д����ʵ�����Ҵ�������Ӧ�Ļ�ѧ����ʽ��_______________________.
(2)Ӧѡ�õ�װ����________(�����)��
(3)��ѡ��װ�õ�����˳��Ӧ��(����ӿڵ���ĸ�����ӽ���ʡ��)________��
(4)������ҽ������һ��ȫ������������ͨ�����г���������ˮ���Ҵ���Ҫ���������к��е�����ˮ��Ӧѡ�ú����Լ�?��ʲô����?(�Լ������������Լ���ѡ)��________��
�𰸣�
������
��ʾ��
������
| (1)2Na+2CH3CH2OH==2CH3CH2ONa+H2����(2)�ݢ٢ۣ�(3)GABE��(4)ȡ�����Լ�������ˮCuSO4���������������ˮ��������ˮ��
|
��ʾ��
| (1)���Ѻ��Ҵ��ķ�Ӧ��ͬ�ҿ��Զ��������2����3��ͬ��������װ�ã���Һ����ȡ����������������4��ѡ����ˮ��ɫ�����ʼ���ˮ
|

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
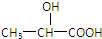 �����Ҵ��Ļ���ﹲ1mol����ȫȼ������54gˮ��56LCO2����״���²ⶨ���������������������ʵ���Ϊ��������
�����Ҵ��Ļ���ﹲ1mol����ȫȼ������54gˮ��56LCO2����״���²ⶨ���������������������ʵ���Ϊ��������