��Ŀ����
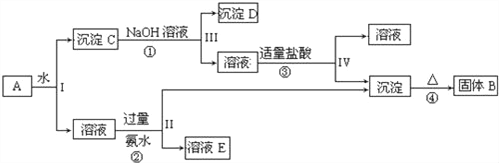
����Ŀ��ij��ɫ��Һ�п��ܺ���Na+��NH4+��Ba2+ ��Cu2+��SO42-��SO32-��Cl-��Br-��CO32-�е������֣�Ϊ�������к��е����ӣ���������ʵ�飺
��ȡ10 mL��Һ������������ˮ��������������ټ���CCl4��Һ�ֲ㣬�²�Ϊ�Ⱥ�ɫ��
�ڷ�Һ�������ϲ���Һ��������BaCl2��HCl��Һ��������ɫ����2.33g��
����ȡ10mLԭ��Һ�����������Ũ����������Һ�����ȣ��ռ�����״����448mL���塣���й���ԭ��Һ��˵����ȷ����
A. �϶�����NH4+��Cl-��Br- B. �Ƿ����Na+��Ҫͨ����ɫ��Ӧ��ȷ��
C. SO42-��SO32-���ٺ���һ�� D. �϶�������Ba2+��Cu2+��SO32-��CO32-
���𰸡�C
����������ɫ��Һ�в�������ɫ��Cu2+������������������ˮ���������������֪ԭ��Һ�в�����CO32-���ټ���CCl4��Һ�ֲ㣬�²�Ϊ�Ⱥ�ɫ��˵������Br-���������ϲ���Һ��������BaCl2��HCl��Һ��������ɫ����2.33g���ó���Ϊ���ᱵ��˵��ԭ��Һ�к���SO42-��SO32-�е�����һ�����ӣ���һ��������Ba2+����������֪���ɵ�����Ϊ�����������ʵ���Ϊ�� ![]() =0.02mol��˵��ԭ��Һ�к���0.02molNH4+��2.33g���ᱵ�����ʵ���Ϊ��
=0.02mol��˵��ԭ��Һ�к���0.02molNH4+��2.33g���ᱵ�����ʵ���Ϊ�� ![]() =0.01mol��������Һ�к��������ӣ����ݵ���غ��֪ԭ��Һ�и���ɵ����ʵ���һ������0.02mol���������ֻ��0.02mol��˵����Һ��һ��������Na+�����ݷ�����֪����Һ��һ�����е�����Ϊ��Na+��NH4+��Br-��SO42-��SO32-�е�����һ�����ӣ�һ�������ڵ�����Ϊ��Ba2+��Cu2+��CO32-������ȷ��������ΪCl-��A���϶�����NH4+��Br-����ȷ���Ƿ���Cl-����A����B�����ݷ�����֪����Һ��һ�����������ӣ�����Ҫͨ����ɫ��Ӧ���飬��B����C�����ݷ�����֪��SO42-��SO32-���ٺ���һ�֣���C��ȷ��D���϶�������Ba2+��Cu2+��CO32-�����ܺ���SO32-����D����ѡC��
=0.01mol��������Һ�к��������ӣ����ݵ���غ��֪ԭ��Һ�и���ɵ����ʵ���һ������0.02mol���������ֻ��0.02mol��˵����Һ��һ��������Na+�����ݷ�����֪����Һ��һ�����е�����Ϊ��Na+��NH4+��Br-��SO42-��SO32-�е�����һ�����ӣ�һ�������ڵ�����Ϊ��Ba2+��Cu2+��CO32-������ȷ��������ΪCl-��A���϶�����NH4+��Br-����ȷ���Ƿ���Cl-����A����B�����ݷ�����֪����Һ��һ�����������ӣ�����Ҫͨ����ɫ��Ӧ���飬��B����C�����ݷ�����֪��SO42-��SO32-���ٺ���һ�֣���C��ȷ��D���϶�������Ba2+��Cu2+��CO32-�����ܺ���SO32-����D����ѡC��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�