��Ŀ����
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ
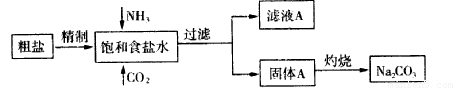
��֪:��ʳ��ˮ��ͨ��NH3��CO2�����ͷ�ӦΪNaCl��NH3��CO2��H2O NaHCO3����NH4Cl,��ش��������⣺
NaHCO3����NH4Cl,��ش��������⣺
��1�������к��е�����������Ca2+��Mg2+��SO42-�ȡ�
���Ƴ��ӵIJ���˳��a��_______��________��________��b(����ĸ��ţ���
a�������ܽ⣬��ȥ������b�����������pH��c������Ba(OH)2��Һ��d������Na2CO3��Һ��e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2��������_____________________��
��2�����չ���A��Na2CO3��_____����ĸ��ţ��н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4+�ķ�����__________________________________________________________��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH=13��Na+��K+����Һ�м�������NH4HCO3��ʹpH���ͣ���Ӧ�����ӷ���ʽ____________________________________��
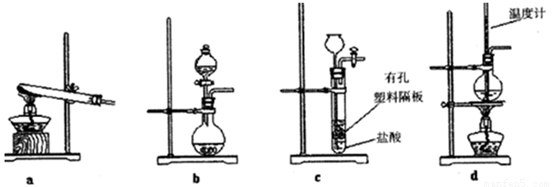
��3����ͼװ���г�����ʵ�����Ʊ�CO2����_____(����ĸ��ţ�����bʾ���װ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ�______(���Լ����ƣ�����ƿ�ڿɼ���Ĺ����Լ�__________�����Լ����ƣ���
��4��һ����Ȼ���ɷ���aNa2CO3��bNa2CO3��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ����������������ѡ�����ʵ�鷽����ȫ����ѡ����Լ���1.0mol/LH2SO4��Һ��1.0mol/L BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1gһ������Ȼ�����Ʒ��������������ˮ�С�
��_________________________________________________________________��
��_________________________________________________________________��
�ܼ�����Ȼ����к�Na2CO3������������
����16�֣���1��c d e��2�֣���NH3������ˮ�������������ܽ�Ȳ����CO2��2�֣�
��2�� a ��1�֣���ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��2�֣���NH4++HCO3-+2OH-��NH3��H2O+CO32-+H2O ��2�֣�
��3��b c ��2�֣�Ũ��ˮ( 1��)����ʯ�ң���NaOH������ʯ�ң�( 1��)
��4�� �ڼ�������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ����1�֣�
�۹��ˡ�ϴ�ӡ����������������2�֣�
��������
�����������1��Ca2����̼���Ƴ�ȥ��Mg2����OH����ȥ��SO42����Ba2����ȥ�������������ữ�������ڹ�����Ba2��Ҫ��̼��������������̼���Ʊ�����������룬������ȷ�IJ���˳����acdeb������NH3������ˮ��CO2��ˮ�е��ܽ�Ȳ���������ͨ�백�������������ܽ�Ȳ����CO2��
��2�����������������н��У���˴�ѡa����������ܺ�ǿ�Ӧ���ɰ��������Կ���ͨ�����鰱��������NH4��,����ȷ�IJ�����ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+������NH4+��HCO3-��OH������Ӧ���Ӷ�ʹ��Һ��pH���ͣ���Ӧ�����ӷ���ʽ��NH4++HCO3-+2OH-��NH3��H2O+CO32-+H2O��
��3��ʵ������ȡCO2���õ���ʯ��ʯ�����ᷴӦ���ҷ�Ӧ����Ҫ���ȣ�����װ�����ʺ���ȡCO2����ѡ��bc���ڰ�ˮ�д���ƽ��NH3��H2O NH3��H2O
NH3��H2O NH4����OH�������Կ�����Ũ��ˮ�м�����ʯ�ң���NaOH������ʯ�ң����Ʊ�����������Һ©����ʢ�ŵ���Ũ��ˮ����ƿ�ڿɼ���Ĺ����Լ���ʯ�ң���NaOH������ʯ�ң���
NH4����OH�������Կ�����Ũ��ˮ�м�����ʯ�ң���NaOH������ʯ�ң����Ʊ�����������Һ©����ʢ�ŵ���Ũ��ˮ����ƿ�ڿɼ���Ĺ����Լ���ʯ�ң���NaOH������ʯ�ң���
��4������̼�����ܺ�ϡ���ᷴӦ����CO2���壬��CO2�ܺͳ����ʯ��ˮ��Ӧ����̼��Ƴ�������˿���ͨ������̼��Ƶ�����������̼���Ƶ�����������ȷ�IJ����Ǣڼ�������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ���۹��ˡ�ϴ�ӡ��������������
���㣺������ε��ᴿ��̼���Ƶ��Ʊ�������ѡ��CO2�Ͱ������Ʊ���NH4+�����Լ����ʺ����ⶨ��ʵ�鷽����Ƶ�

�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100 gˮ����
�¶� �ܽ�� �� | 0 �� | 10 �� | 20 �� | 30 �� | 40 �� | 50 �� | 60 �� | 100 �� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
NH4C | l29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�٣�35�� NH4HCO3���зֽ�
��ش�
��1����Ӧ�¶ȿ�����30��35 �棬����Ϊ������35 �棬��__________________________��������30 �棬��______________________________________��Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ__________________________________________________________��
��2��������Ϻ�������30���ӣ�Ŀ����______________________________________�����ú�ֻ����NaHCO3�����ԭ����___________________________________________��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ_______________________________________���ʣ��Ի�ѧʽ��ʾ����
��3���������õ�ĸҺ�к���______________________________________���Ի�ѧʽ��ʾ���������___________________��������һ��������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4Cl��
��4�����Դ����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷW g��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc( mol��L-1)��HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ��ָʾ![]() +H+====
+H+====![]() ��Ӧ���յ㣩������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ�����ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��
��Ӧ���յ㣩������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ�����ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��
w(NaHCO3)=___________________��
?