��Ŀ����
 �����»�ѧ��Ӧ��2A��g��+B��g��?2C��g������H��0��
�����»�ѧ��Ӧ��2A��g��+B��g��?2C��g������H��0����1������4molA��2molB��2L�������л�ϣ���2s����C��Ũ��Ϊ0.6mol?L-1��������A��ʾ��ƽ����Ӧ����Ϊ
��2������amolA��bmolB����һ�ܱ������У��ﵽƽ��ʱ���ǵ����ʵ������㣺n��A��+n��B��=n��C������A��ת����Ϊ
��3������4molA��2molB��������ɱ�ĵ�ѹ�����У�һ���¶��´ﵽƽ��״̬��������������ʵ���Ϊ4.2mol����ʱ�����������C���������Ϊ
��4����ͼ��һ�������¸÷�Ӧ�����У���ϵ�ڸ��� ��Ũ�ȵı仯�������Ӧ����ƽ��״̬��ʱ����
��������1�����ݷ�Ӧ2A��g��+B��g��?2C��g����������ʽ������������ʵ����ʵ���Ũ�ȣ����û�ѧ��Ӧ����V=
�����
��2�����ݷ�Ӧ2A��g��+B��g��?2C��g����������ʽ������������ʵ����ʵ���Ũ�ȣ�����A��ת���ʹ�ʽ���㣻
��3���跴Ӧ��AΪXmol�����ݷ�Ӧ2A��g��+B��g��?2C��g����������ʽ�����X������������ʵ����ʵ�����Ȼ��������������C�����ʵ������������������
���ݷ�Ӧ��Ũ������ƽ�������ƶ������
�������������Ũ�ȡ��¶ȡ�ѹǿ���Ի�ѧƽ���Ӱ����������
��4��ƽ��״̬ʱ�����ʵ�Ũ�ȱ��ֲ��䣬����ͼ�������t2ʱ���߷����仯��AB���٣�C���ӣ�˵��ƽ�����ƣ�t4ʱ��B��Ũ������0.1mol?L-1��Ȼ����С��t4ʱ����Ũ��˲�䲻�䣬ƽ�����ƣ�AŨ�ȼ�С��CŨ������
| ��C |
| ��t |
��2�����ݷ�Ӧ2A��g��+B��g��?2C��g����������ʽ������������ʵ����ʵ���Ũ�ȣ�����A��ת���ʹ�ʽ���㣻
��3���跴Ӧ��AΪXmol�����ݷ�Ӧ2A��g��+B��g��?2C��g����������ʽ�����X������������ʵ����ʵ�����Ȼ��������������C�����ʵ������������������
���ݷ�Ӧ��Ũ������ƽ�������ƶ������
�������������Ũ�ȡ��¶ȡ�ѹǿ���Ի�ѧƽ���Ӱ����������
��4��ƽ��״̬ʱ�����ʵ�Ũ�ȱ��ֲ��䣬����ͼ�������t2ʱ���߷����仯��AB���٣�C���ӣ�˵��ƽ�����ƣ�t4ʱ��B��Ũ������0.1mol?L-1��Ȼ����С��t4ʱ����Ũ��˲�䲻�䣬ƽ�����ƣ�AŨ�ȼ�С��CŨ������
����⣺��1����ʼA��Ũ��Ϊ
=2mol/L��B��Ũ��Ϊ
=1mol/L����
2A��g��+B��g��?2C��g��
��ʼ��mol/L����2 1 0
�仯��mol/L����0.6 0.3 0.6
2sʱ��mol/L����1.4 0.7 0.6
2sʱ����B��Ũ��Ϊ0.7 mol/L��������A��ʾ��ƽ����Ӧ����Ϊ
=0.3mol/��L?s����
�ʴ�Ϊ��0.3mol/��L?s����0.7 mol/L��
��2����μӷ�Ӧ��AΪxmol
2A��g��+B��g��?2C��g��
��ʼ��mol����a b 0
�仯��mol����x
x x
ƽ��ʱ��mol����a-x b-
x x
�ﵽƽ��ʱ���ǵ����ʵ������㣺n��A��+n��B��=n��C������a-x+b-
x=x������x=
��a+b��������A��ת����Ϊ
��100%=
��100%��
�ʴ�Ϊ��
��100%��
��3���跴Ӧ��AΪ2Xmol����
2A��g��+B��g��?2C��g��
��ʼ��mol�� 4 2 0
�仯��mol����2X X 2X
ƽ�⣨mol����4-2X 2-X 2X
������������ʵ���Ϊ4-2X+2-X+2X=4.2�����X=1.8��
��ƽ��ʱA��B��C�����ʵ����ֱ�Ϊ0.4mol��0.2mol��3.6mol�����������C�����ʵ�������Ϊ
��100%=85.7%�������������C���������Ϊ85.7%��
��ͨ������B���壬��Ӧ���Ũ������ƽ�������ƶ�����ϵ��A�����ʵ������٣�
��ҪʹA�����ʵ����ٴﵽ��ԭƽ��״̬��ͬ����ƽ���ͨ������ƽ�������ȵķ����淴Ӧ�ƶ����ѹƽ������������淴Ӧ�ƶ���ͨ������A���壬ƽ�������ƶ���
�ʴ�Ϊ��85.7%����С�����»��Сѹǿ�����A���壻
��4��ƽ��״̬ʱ�����ʵ�Ũ�ȱ��ֲ��䣬����ͼ�������֪t1-t2 t3-t4Ϊƽ��״̬��t2ʱ���߷����仯��AB���٣�C���ӣ�˵��ƽ�����ƣ����Ҹ�������б�ʱ仯��֪��t2ʱ���ʼ�С�����Ըı������ǽ����¶ȣ�t4ʱ��B��Ũ������0.1mol?L-1��Ȼ����С��t4ʱ����Ũ��˲�䲻�䣬ƽ�����ƣ�AŨ�ȼ�С��CŨ�������ݷ����м�������ϵȷ�������ʵı仯������������Ϊ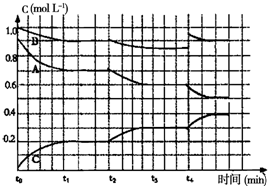 ��
��
�ʴ�Ϊ��t1-t2 t3-t4 �������¶ȣ� ��
��
| 4mol |
| 2L |
| 2mol |
| 2L |
2A��g��+B��g��?2C��g��
��ʼ��mol/L����2 1 0
�仯��mol/L����0.6 0.3 0.6
2sʱ��mol/L����1.4 0.7 0.6
2sʱ����B��Ũ��Ϊ0.7 mol/L��������A��ʾ��ƽ����Ӧ����Ϊ
| 0.6mol/L |
| 2s |
�ʴ�Ϊ��0.3mol/��L?s����0.7 mol/L��
��2����μӷ�Ӧ��AΪxmol
2A��g��+B��g��?2C��g��
��ʼ��mol����a b 0
�仯��mol����x
| 1 |
| 2 |
ƽ��ʱ��mol����a-x b-
| 1 |
| 2 |
�ﵽƽ��ʱ���ǵ����ʵ������㣺n��A��+n��B��=n��C������a-x+b-
| 1 |
| 2 |
| 2 |
| 5 |
| ||
| a |
| 2(a+b) |
| 5a |
�ʴ�Ϊ��
| 2(a+b) |
| 5a |
��3���跴Ӧ��AΪ2Xmol����
2A��g��+B��g��?2C��g��
��ʼ��mol�� 4 2 0
�仯��mol����2X X 2X
ƽ�⣨mol����4-2X 2-X 2X
������������ʵ���Ϊ4-2X+2-X+2X=4.2�����X=1.8��
��ƽ��ʱA��B��C�����ʵ����ֱ�Ϊ0.4mol��0.2mol��3.6mol�����������C�����ʵ�������Ϊ
| 3.6mol |
| 4.2mol |
��ͨ������B���壬��Ӧ���Ũ������ƽ�������ƶ�����ϵ��A�����ʵ������٣�
��ҪʹA�����ʵ����ٴﵽ��ԭƽ��״̬��ͬ����ƽ���ͨ������ƽ�������ȵķ����淴Ӧ�ƶ����ѹƽ������������淴Ӧ�ƶ���ͨ������A���壬ƽ�������ƶ���
�ʴ�Ϊ��85.7%����С�����»��Сѹǿ�����A���壻
��4��ƽ��״̬ʱ�����ʵ�Ũ�ȱ��ֲ��䣬����ͼ�������֪t1-t2 t3-t4Ϊƽ��״̬��t2ʱ���߷����仯��AB���٣�C���ӣ�˵��ƽ�����ƣ����Ҹ�������б�ʱ仯��֪��t2ʱ���ʼ�С�����Ըı������ǽ����¶ȣ�t4ʱ��B��Ũ������0.1mol?L-1��Ȼ����С��t4ʱ����Ũ��˲�䲻�䣬ƽ�����ƣ�AŨ�ȼ�С��CŨ�������ݷ����м�������ϵȷ�������ʵı仯������������Ϊ
 ��
���ʴ�Ϊ��t1-t2 t3-t4 �������¶ȣ�
 ��
�����������⿼�黯ѧ��Ӧ�����йؼ����Լ���ѧƽ���ƶ�����ƽ������ͼ�ȣ��ѶȽϴ�ע����������ʽ�����ʾ����������������صļ��㣮

��ϰ��ϵ�д�
�����Ŀ

 2C��g������H<0��
2C��g������H<0��