��Ŀ����
�л���W(C3H6O3)����NaHCO3��Ӧ�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1��
��1��W�Ľṹ��ʽ������������������������
��2��W�Ĺ�ҵ�ϳ�·������ͼ��ʾ��

��֪����.A��B��C��D��W�����к�����̼ͬԭ������
��.![]()
��д��A�Ľṹ��ʽ������������������������
��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ������������������������
��D��NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ������������������
��C��ͬ���칹���У��ܹ���Ag(NH3)2OH��Һ��Ӧ�������������֡�
��3����ҵ��Ҳ��������ͼ��ʾ�ϳ�·������W��
![]()
��֪��![]()
���ںϳ�·�߿�ͼ��������Ӧ�л���Ľṹ��ʽ��
��4��W��һ�������£���Ӧ���ɱ�����(C6H8O4)(����Ԫ���ṹ)���÷�Ӧ�Ļ�ѧ����ʽ��(�л���д�ṹ��ʽ) ��
��������һ�������¾ۺ����ɾ۱�����(![]() )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
��
��1��![]()
��2����CH3CH2CH2OH��
��CH3CH2CHO��2Cu(OH)2![]() CH3CH2COOH��Cu2O��2H2O
CH3CH2COOH��Cu2O��2H2O
��![]()
��3
��3��CH2��CH2��CH3CH2OH��CH3CHO
��4��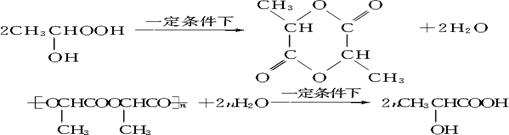

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�



 )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��

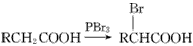

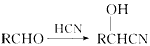
 )���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��
)���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ��