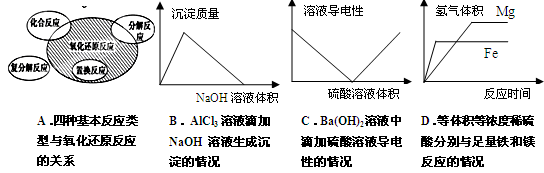
��Ŀ����
������ط�Ӧ�����ӷ���ʽ��д�������
| A����ǿ����Һ��NaClO��Fe(OH)3��Ӧ����Na2FeO4��3ClO��+4OH��+2Fe(OH)3��3Cl��+5H2O+2FeO42�� |
| B������SO2ͨ�뱽������Һ�У�2C6H5O��+SO2+H2O��2C6H5OH+SO32�� |
| C��0.01mol��L��1NH4Al(SO4)2��Һ��0.02mol��L��1Ba(OH)2��Һ�������ϣ� NH4��+Al3��+2SO42��+2Ba2��+4OH����2BaSO4��+Al(OH)3��+NH3��H2O |
| D������SO2ͨ��NaClO��Һ�У�SO2 + ClO��+ OH���� SO42�� + Cl�� + H+ |
D
��������������ж����ӷ���ʽ��ȷ���ķ���һ���ǣ���1����鷴Ӧ�ܷ�������2����鷴Ӧ��������Ƿ���ȷ����3���������ʲ���Ƿ���ȷ����4������Ƿ�����غ��ϵ���磺�����غ�͵���غ�ȣ�����5������Ƿ����ԭ��ѧ����ʽ��A����ǿ����Һ��NaClO��Fe(OH)3����������ԭ��Ӧ����Na2FeO4���Ȼ��ƺ�ˮ�����ӷ���ʽΪ3ClO��+4OH��+2Fe(OH)3��3Cl��+5H2O+2FeO42����A��ȷ��B�������������ǿ�ڱ��ӵģ��������SO2ͨ�뱽������Һ�����ɱ��Ӻ��������ƣ����ӷ���ʽΪ2C6H5O��+SO2+H2O��2C6H5OH+SO32����B��ȷ��C��0.01mol��L��1NH4Al(SO4)2��Һ��0.02mol��L��1Ba(OH)2��Һ��������ʱ��OH��������Al3�������������������ɫ������Ȼ������NH4���������һˮ�ϰ�����������ᱵ��ɫ�������ɣ���Ӧ�����ӷ���ʽΪ��NH4����Al3����2SO42����2Ba2����4OH����2BaSO4����Al(OH)3����NH3��H2O��C��ȷ��D��SO2���л�ԭ�ԣ��������ƾ��������ԣ��������SO2ͨ��NaClO��Һ�з���������ԭ��Ӧ���������ơ��Ȼ��ƣ���Ӧ�����ӷ���ʽΪSO2 + ClO��+ H2O�� SO42�� + Cl�� + 2H+��D����ȷ����ѡD��
���㣺�������ӷ���ʽ�������ж�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д������£����и���������ָ����Һ��һ���ܴ����������
| A����ʹ��̪������Һ��Na+��Ba2+��NO3����Cl�� |
| B������0.1 mol��L-1 Fe3������Һ�У�K����Mg2����I����SO42�� |
| C�����ܽ�Al(OH)3����Һ��NH4+��K+��SO42����HCO3�� |
| D��c(Al3+)="0.5" mol��L-1����Һ�У�Na+��K+��[Al(OH)4]����SO42�� |
���и�����������Һ���ܹ�����ͨ����������Ӧ��������ܴ������ڵ���
| A��Na+��Ba2+��HSO3-��I-�������� |
| B��Ca2+��NO3-��Na+��Cl-���������� |
| C��Fe3+��SO42-��CO32-��NO3- �������� |
| D��Na+��K+��HCO3-��Cl-��������̼�� |
��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӡ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ� ��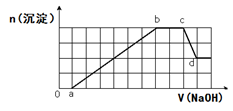
| A��d����Һ�к��е�����ֻ��Na2SO4 |
| B��ab�η��������ӷ�ӦΪ��Al3++3OH-= Al(OH)3��, Mg2++2OH-= Mg(OH)2�� |
| C��ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+��Na+ |
| D��ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1:1 |
ij��Һ���ܺ���Cl�C��SO42�C��CO32�C��NH4+��Fe2+��Al3+��K+��ȡ����Һ100 mL���������NaOH��Һ�����ȣ��õ�0. 02 mol���壬ͬʱ�������������ˡ�ϴ�ӡ����գ��õ�1.6 g����ɫ���壻��������Һ�м�������BaCl2��Һ���õ�4.66 g����������ij������ɴ˿�֪ԭ��Һ��
| A�����ٴ���4������ |
| B��Cl�C һ�����ڣ���c(Cl )��0.4 mol/L |
| C��SO42�C��NH4+��һ�����ڣ�Cl�C���ܲ����� |
| D��CO32�C��Al3+ һ�������ڣ�K+���ܴ��� |
ij��Һ�д��ڽ϶��H+��SO42-��NO3-������Һ�л����ܴ������ڵ���������
| A��Mg2+��Ba2+��Br- | B��Al3+��CH3COO-��Cl- |
| C��Mg2+��Cl-��Fe2+ | D��Na+��NH4+��Cl- |
����������������ӷ���ʽ����ȷ����
| A��������ϡ���ᣬ��Һ���: 3 Fe +8H+ +2NO3-="3" Fe2++2NO+4H2O |
| B��FeBr2��Cl2���ʵ���֮��Ϊ1��1ʱ:2Fe2++2Br-+2Cl2=2Fe3++4C1-+Br2 |
| C����������Һ�еμ�����������Һ��SO42-��ȫ����:A13++2SO42-+2Ba2++4OH-=2BaSO4��+A1O2-+2H2O |
| D��Ư����Һ�м��Ȼ�����Һ�����������ɫ����:Fe3++3C1O-+3H2O=Fe(OH)3��+3HC1O |
����״̬�����ʣ����ܵ��������ڵ���ʵ���
| A��KCl��Һ | B��Һ̬HCl | C�����ڵ�NaOH | D��������Һ |