��Ŀ����
(12��)ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500 g��
�������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5 min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
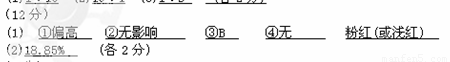
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����________(�ƫ����ƫС������Ӱ�족)��
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______ _ ��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����ָ̪ʾ����______________ɫ���____________ɫ��
(2)�ζ�������±���ʾ��
|
����� |
������Һ�����/mL |
����Һ����� |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 |
1.02 |
21.03 |
|
2 |
25.00 |
2.00 |
21.99 |
|
3 |
25.00 |
0.20 |
20.20 |
��NaOH����Һ��Ũ��Ϊ0.101 0 mol/L�������Ʒ�е�����������Ϊ________��
����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(9��)ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣮij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500 g.
�������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5 min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
������ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
������ƿ������ˮϴ�Ӻ�ˮδ������������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�����ζ�ǰ����ʽ�ζ��ܼ��촦�����ݣ����ڵζ���������ʧ��������Ʒ�е�����������________ (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�����ζ�ǰ�����ӵ����ƶ�ȡ�˼�ʽ�ζ��ܵĶ������ζ��������ȷ��������Ʒ�е�����������________ (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�ݵζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ζ��ﵽ�յ�ʱ����ָ̪ʾ����______ɫ���________ɫ��
(2)�ζ�������±���ʾ��
|
����� |
������Һ�����/mL |
����Һ����� |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 |
1.02 |
21.03 |
|
2 |
25.00 |
2.00 |
21.99 |
|
3 |
25.00 |
0.20 |
20.20 |
��NaOH����Һ��Ũ��Ϊ0.10 mol/L�������Ʒ�е�����������Ϊ________��
(12��)ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500 g��
�������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250mL��ƿ�У�����10 mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����________(�ƫ����ƫС������Ӱ�족)��
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______ _ ��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����ָ̪ʾ����______________ɫ���____________ɫ��
(2)�ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
��NaOH����Һ��Ũ��Ϊ0.101 0 mol/L�������Ʒ�е�����������Ϊ________��
 ɫ���____________ɫ��
ɫ���____________ɫ��