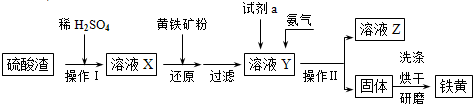
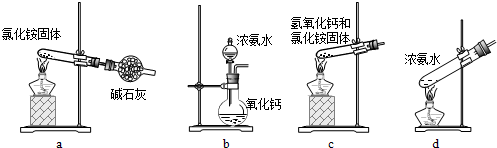
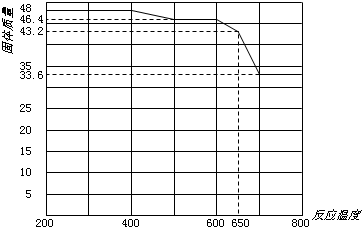
��Ŀ����
����ʵ���������������ʵ��������˵����ȷ����(����)
| A�������²ⶨ������Ħ���������22.4 L��mol��1 |
| B��100 mL 0.1 mol��L��1NaOH��Һ��100 mL 0.1mol��L��1CH3COOH��Һ��Ӧ�ų�����������573 J |
| C������1.0 mol��L��1NaCl��Һ����ʱ��������ƿ�Ŀ̶��ߵ���������ҺŨ��ƫ�� |
| D���к͵ζ�ʱ����ƿ����ˮ��ע�����Һ���������ҺŨ�ȼ�С |
A
�������������ѡ��A�������µ��¶ȴ���0 �棬��ѹǿ����������ʵ���һ��ʱ���¶�Խ�߲�������Խ��ѡ��B��CH3COOH�����ᣬ������Ҫ���ȣ������ֵӦС��573 J��ѡ��C������ʱ��������ƿ�Ŀ̶���ʱ������ˮ����������������ҺŨ��ƫ�͡�ѡ��D����ƿ���Ƿ���ˮ�Բⶨ���û��Ӱ�죬��˴�ѡA��
���㣺����ʵ�����������ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ������������������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������
����Ĺؼ�����ȷʵ��ԭ����Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
�����Ŀ