ΧβΡΩΡΎ»ί
(―ΓΉωΧβ)ΓΨΈο÷ ΫαΙΙ”κ–‘÷ ΡΘΩιΓΩXΓΔYΓΔZΓΔW «‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΒΡΕΧ÷ήΤΎ‘ΣΥΊΘ§«“ΜΞ≤ΜΆ§ΉεΘ§Τδ÷–÷Μ”–“Μ÷÷ΈΣΫπ τΘ§XΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣnsnnpnΘ§ZΒΡΉνΆβ≤ψΒγΉ” ΐ «ΤδΒγΉ”≤ψ ΐΒΡ2±ΕΘ§ Y‘≠Ή””κZ‘≠Ή”ΒΡΉνΆβ≤ψΒγΉ” ΐ÷°ΚΆΈΣ9Θ§«“YΚΆWΒΞ÷ ΕΦΩ…”κ…’Φν»ή“ΚΖ¥”ΠΘ§«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
(1)YΓΔZΓΔWΒΡ‘≠Ή”ΑκΨΕ”…¥σΒΫ–ΓΒΡΥ≥–ρ «________________(Χν‘ΣΥΊΖϊΚ≈)
(2)XZ2ΒΡΒγΉ” Ϋ «________________Θ§Ω’ΦδΫαΙΙΈΣ________________Θ§–Έ≥…ΨßΧε ± τ”Ύ________________ΨßΧεΓΘ
(3)YΒΡΦέΒγΉ”≈≈≤Φ Ϋ «________________Θ§ΙΛ“Β…œ…ζ≤ζΒΞ÷ YΒΡ‘≠άμ «________________(”ΟΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ±μ Ψ)
(4) ‘”Οά’œΡΧΊΝ–‘≠άμΫβ Ά”Ο±ΞΚΆ ≥―ΈΥ° ’Φ·WΒΞ÷ ΒΡ‘≠άμ________________ΓΘ
(1)AlΘΨSΘΨCl
(2)![]() ÷±œΏ–Έ Ζ÷Ή”
÷±œΏ–Έ Ζ÷Ή”
(3)3s23p1
2Al2O3 ![]() 4Al+3O2Γϋ
4Al+3O2Γϋ
(4)Cl2+H2O ![]() H++Cl-+HClOΘ§±ΞΚΆ ≥―ΈΥ°Cl-≈®Ε»¥σΘ§ Ι…œ ωΤΫΚβΡφœρ“ΤΕ·Θ§ΫΒΒΆΝΥCl2‘ΎΥ°÷–ΒΡ»ήΫβΕ»
H++Cl-+HClOΘ§±ΞΚΆ ≥―ΈΥ°Cl-≈®Ε»¥σΘ§ Ι…œ ωΤΫΚβΡφœρ“ΤΕ·Θ§ΫΒΒΆΝΥCl2‘ΎΥ°÷–ΒΡ»ήΫβΕ»
ΫβΈωΘΚXΓΔYΓΔZΓΔWΥΡ‘ΣΥΊΖ÷±πΈΣCΓΔAlΓΔSΓΔCl
(1)AlΘΨSΘΨCl
(2)XZ2ΒγΉ” ΫΘΚ![]()
Ω’ΦδΙΙ–ΆΈΣ÷±œΏ–ΈΘ§ΨßΧε τ”ΎΖ÷Ή”ΨßΧε
(3)AlΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ3s23p1Θ§ΙΛ“Β…œ÷Τ¬Ν‘≠άμ
2Al2O3 ![]() 4Al+3O2Γϋ
4Al+3O2Γϋ
(4)Cl2»ή”ΎΥ°Θ§¥φ‘ΎΤΫΚβCl2+H2O ![]() H++Cl-+HClOΘ§±ΞΚΆ ≥―ΈΥ°÷–Θ§Cl-≈®Ε»Ϋœ¥σΘ§ Ι…œ ωΤΫΚβΡφœρ“ΤΕ·Θ§ΫΒΒΆΝΥCl2‘ΎΥ°÷–ΒΡ»ήΫβΕ»ΓΘ
H++Cl-+HClOΘ§±ΞΚΆ ≥―ΈΥ°÷–Θ§Cl-≈®Ε»Ϋœ¥σΘ§ Ι…œ ωΤΫΚβΡφœρ“ΤΕ·Θ§ΫΒΒΆΝΥCl2‘ΎΥ°÷–ΒΡ»ήΫβΕ»ΓΘ

 ”≈―ßΟϊ ΠΟϊΧβœΒΝ–¥πΑΗ
”≈―ßΟϊ ΠΟϊΧβœΒΝ–¥πΑΗ(A)ΓΨΈο÷ ΫαΙΙ”κ–‘÷ ΓΩ
œ¬±μ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷ΓΘ±μ÷–ΥυΝ–ΒΡΉ÷ΡΗΖ÷±π¥ζ±μΡ≥“Μ÷÷Μ·―ß‘ΣΥΊΓΘ

(1)T3+ΒΡΚΥΆβΒγΉ”≈≈≤Φ Ϋ «____________ΓΘ
(2)QΓΔRΓΔMΒΡΒΎ“ΜΒγάκΡή”…¥σΒΫ–ΓΒΡΥ≥–ρ «___________________(”Ο‘ΣΥΊΖϊΚ≈±μ Ψ)ΓΘ
(3)œ¬Ν–”–ΙΊ…œ ω‘ΣΥΊΒΡΥΒΖ®÷–Θ§’ΐ»ΖΒΡ «______________________(Χν–ρΚ≈)ΓΘ
ΔΌGΒΞ÷ ΒΡ»έΒψΗΏ”ΎJΒΞ÷ Θ§ «“ρΈΣGΒΞ÷ ΒΡΫπ τΦϋΫœ«Ω
ΔΎJ±»XΜνΤΟΘ§Υυ“‘JΩ…“‘‘Ύ»ή“Κ÷–÷ΟΜΜ≥ωX
ΔέΫΪJ
ΔήRE3Ζ–ΒψΗΏ”ΎQE4Θ§÷ς“Σ «“ρΈΣ«Α’ΏœύΕ‘Ζ÷Ή”÷ ΝΩΫœ¥σ
Δί“ΜΗωQ2E4Ζ÷Ή”÷–Κ§”–ΈεΗωΠ“ΦϋΚΆ“ΜΗωΠ–Φϋ
(4)Φ”ΡΟ¥σΧλΈΡΧ®‘ΎΧΪΩ’ΖΔœ÷ΝΥEQ9RΘ§“―÷ΣΖ÷Ή”÷–Υυ”–‘≠Ή”Ψυ–Έ≥…8ΒγΉ”Μρ2ΒγΉ”Έ»Ε®ΫαΙΙΘ§ «÷±œΏ–ΈΖ÷Ή”Θ§≤Μ¥φ‘Ύ≈δΈΜΦϋΓΘ–¥≥ωΤδΫαΙΙ ΫΘΚ_________________ΓΘ
(5)G”κRΒΞ÷ ÷±Ϋ”Μ·Κœ…ζ≥…“Μ÷÷άκΉ”Μ·ΚœΈοG3RΓΘΗΟΨßΧεΨΏ”–άύΥΤ ·ΡΪΒΡ≤ψΉ¥ΫαΙΙΓΘΟΩ≤ψ÷–Θ§G‘≠Ή”ΙΙ≥…ΤΫΟφΝυ±Ώ–ΈΘ§ΟΩΗωΝυ±Ώ–ΈΒΡ÷––Ρ”–“ΜΗωR‘≠Ή”ΓΘ≤ψ”κ≤ψ÷°ΦδΜΙΦ–‘”“ΜΕ® ΐΝΩΒΡ‘≠Ή”ΓΘ«κΈ ’β–©Φ–‘”ΒΡ‘≠Ή””ΠΗΟ «____________(ΧνGΜρRΒΡ‘ΣΥΊΖϊΚ≈)ΓΘ

(B)ΓΨ Β―ιΜ·―ßΓΩ
Ρ≥Ή Νœœ‘ ΨΘ§Ρή ΙΥΪ―θΥ°Ζ÷ΫβΒΡ¥ΏΜ·ΦΝ”–ΚήΕύ÷÷Θ§…ζΈο¥ΏΜ·ΦΝ(»γ÷μΗΈ)ΓΔάκΉ”–Ά¥ΏΜ·ΦΝ(»γFeCl3)ΚΆΙΧΧε¥ΏΜ·ΦΝ(»γMnO2)Β»ΕΦ «ΫœΚΟΒΡ¥ΏΜ·ΦΝΓΘΡ≥ Β―ι–ΓΉιΆ®Ιΐ≤βΕ®ΥΪ―θΥ°Ζ÷Ϋβ≤ζ…ζΒΡO2ΒΡ―Ι«ΩΘ§ΧΫΨΩΖ÷ΫβΙΐ―θΜ·«βΒΡΉνΦ―¥ΏΜ·ΦΝ“‘ΦΑΧΫΨΩΉνΦ―¥ΏΜ·ΦΝΚœ ΒΡ¥ΏΜ·ΧθΦΰΓΘ
(“Μ)ΧΫΨΩ“ΜΘΚ
Β―ι≤Ϋ÷η
(1)ΆυΉΕ–ΈΤΩ÷–Φ”»κ50 mL 1.5ΘΞΒΡΥΪ―θΥ°
(2)Ζ÷±πΆυΉΕ–ΈΤΩ÷–Φ”
(3)≤…Φ·ΚΆΦ«¬Φ ΐΨίΓΘ
(4)’ϊάμ ΐΨίΒΟ≥ωœ¬±μ
≤ΜΆ§¥ΏΜ·ΦΝΓΑ―Ι«ΩΕ‘ ±Φδ–±¬ Γ±ΒΡ±»Ϋœ
¥ΏΜ·ΦΝ | ÷μΗΈ | ¬μΝε μ | ¬»Μ·Ά≠ | ¬»Μ·Χζ | ―θΜ·Ά≠ | Εΰ―θΜ·ΟΧ |
―Ι«ΩΕ‘ ±ΦδΒΡ–±¬ | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
ΔΌΗΟΓΑΧΫΨΩ“ΜΓ± Β―ιΒΡΟϊ≥Τ «_____________________________________________________ΓΘ
ΔΎΗΟ Β―ιΥυΒΟ≥ωΒΡΫα¬έ «_______________________________________________________ΓΘ
(Εΰ)ΧΫΨΩΕΰΘΚΕΰ―θΜ·ΟΧ¥ΏΜ·ΒΡΉνΦ―¥ΏΜ·ΧθΦΰ
ΗΟ Β―ι–ΓΉιΒΡΆ§―ß‘ΎΫχ––ΧΫΨΩΕΰΒΡ Β―ι ±Θ§ΒΟΒΫΝΥ“ΜœΒΝ–ΒΡΆΦ±μΚΆ ΐΨίΓΘ≤ΈΩ¥œ¬ΆΦΚΆ±μΗώΖ÷±πΜΊ¥πœύΙΊΈ ΧβΓΘ

3%ΒΡΥΪ―θΥ°”κ≤ΜΆ§”ΟΝΩΕΰ―θΜ·ΟΧΒΡ―ΙΝΠΓΣ ±ΦδΆΦ
±μΘΚ≤ΜΆ§≈®Ε»ΒΡΥΪ―θΥ°‘Ύ≤ΜΆ§”ΟΝΩΒΡΕΰ―θΜ·ΟΧΉς”Οœ¬ ’Φ·œύΆ§Ή¥Ωωœ¬Ά§ΧεΜΐO2Υυ–η ±Φδ
MnO2 ±Φδ H2O2 | |||
1.5ΘΞ | 223 s | 67 s | 56 s |
3.0ΘΞ | 308 s | 109 s | 98 s |
4.5ΘΞ | 395 s | 149 s | 116 s |
Ζ÷ΈωΆΦΓΔ±μ÷– ΐΨίΈ“Ο«Ω…“‘ΒΟ≥ωΘΚ
ΔέΆ§≈®Ε»ΒΡΥΪ―θΥ°ΒΡΖ÷ΫβΥΌ¬ ΥφΉ≈Εΰ―θΜ·ΟΧ”ΟΝΩΒΡ‘ωΦ”Εχ_________________Θ§“ρΕχΖ¥”Π ±Φδ_______________ΓΘ
Δή»γΙϊ¥” Β―ιΫαΙϊΚΆΫΎ Γ“©ΤΖΒΡΫ«Ε»ΉέΚœΖ÷ΈωΘ§Ρψ»œΈΣΒ±Έ“Ο«―Γ”Ο3.0%ΒΡΥΪ―θΥ°Θ§Φ”»κ___________ gΒΡΕΰ―θΜ·ΟΧΡή Ι Β―ι–ßΙϊΉνΦ―ΓΘΡψ≈–ΕœΒΡάμ”… «______________________ΓΘ
ΔίΗΟ–ΓΉιΒΡΡ≥Ά§―ßΆ®ΙΐΖ÷Έω ΐΨίΒΟ≥ωΝΥΒ±¥ΏΜ·ΦΝ”ΟΝΩœύΆ§ ±ΥΪ―θΥ°ΒΡ≈®Ε»‘Ϋ–ΓΖ¥”ΠΥΌ¬ ‘ΫΩλΒΡΫα¬έΘ§Ρψ»œΈΣ «Ζώ’ΐ»Ζ____________Θ§ΡψΒΡάμ”… «________________________________ΓΘ
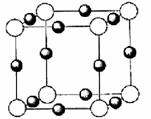
‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΒΡAΓΔBΓΔCΓΔDΓΔEΓΔF(AΓΔBΓΔCΓΔDΓΔEΓΔFΖ÷±π¥ζ±μ‘ΣΥΊΖϊΚ≈)Νυ÷÷ΕΧ÷ήΤΎ‘ΣΥΊΓΘ‘ΣΥΊAΒΡ‘≠Ή”ΑκΨΕ‘ΎΕΧ÷ήΤΎ÷–Ήν–ΓΘ§‘ΣΥΊCΒΡΒΞ÷ ‘ΎΩ’Τχ÷–Κ§ΝΩΉνΕύΘ§D+±»F-…Ό“ΜΗωΒγΉ”≤ψΘ§EΥυ‘Ύ÷ήΤΎΒΡΗς‘ΣΥΊΒΞ÷ Ζ–Βψ±δΜ·»γœ¬ΆΦ(‘ΣΥΊΑ¥‘≠Ή”–ρ ΐΒί‘ωΥ≥–ρΝ§–χ≈≈Ν–)Θ§BΓΔCΝΫ÷÷‘ΣΥΊΖ÷±πΡή”κA–Έ≥…Β»ΒγΉ”ΒΡΦΉΓΔ““ΝΫ÷÷Ζ÷Ή”Θ§«“ΝΫ÷÷Ζ÷Ή”÷–Ης‘≠Ή”ΒΡΗω ΐΦϊœ¬±μΘΚ
Μ·ΚœΈο | ΦΉ | ““ |
‘≠Ή”Ηω ΐ±» | BΓΟA=1ΓΟ4 | CΓΟA=1ΓΟ3 |
(1)‘ΣΥΊEΒΡΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ________________________ΓΘ
(2)–¥≥ωΜ·ΚœΈο““ΒΡΫαΙΙ Ϋ__________________Θ§ΗΟΖ÷Ή”÷–CΒΡ‘≠Ή”ΙλΒάΖΔ…ζΒΡ «____________‘”Μ·ΓΘ
(3)BΓΔC–Έ≥…ΒΡ“Μ÷÷Μ·ΚœΈοX «“Μ÷÷‘≠Ή”ΨßΧεΘ§ΨßΧε÷–BΓΔC‘≠Ή”Ψυ¥οΒΫ8ΒγΉ”Έ»Ε®ΫαΙΙΘ§‘ρXΒΡΜ·―ß ΫΈΣ__________________ΓΘ
(4)D‘ΎF÷–»Φ…’ΒΡ≤ζΈο τ”Ύ______________ΨßΧεΘ§ΤδΨßΧε÷–”κD+ΉνΫϋ«“Β»ΨύάκΒΡF-”–___________ΗωΓΘ