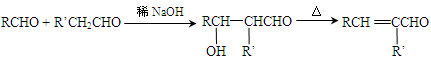
��Ŀ����
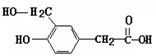
��֪��CH2=CHOH�ڳ����ºܲ��ȶ����Զ�ת��ΪCH3CHO��ij���������ʽΪC10H6O6�������ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫������������������������ˮ��ΪA�ͱ�ͪ�ᣨC3H4O3��

��1�����л�������к��к��������ŵ������� ��
��2��д�����л���NaOH��Һ��Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ�� ��
��3��A�ж���ͬ���칹�壬ͬʱ��������Ҫ���A��ͬ���칹�干�� �֡�
��1mol��ͬ���칹������5molNaOH������Ӧ
�ں˴Ź���������ʾ���ĸ����շ�
��4����ͪ����һ�ֳ����л��ϳ��м��塣��ʯ���ѽ����ɷ�֮һΪԭ�ϣ�ͨ�����������Ƶñ�ͪ�ᣬ�����ϳɸ߾���K��

��д���������ʵĽṹ��ʽ��D�� M�� ��
���ڢ١������У����ڼӳɷ�Ӧ���� ���ñ����д��
�۵ڢڲ��Ļ�ѧ��Ӧ����ʽΪ ��

��1�����л�������к��к��������ŵ������� ��
��2��д�����л���NaOH��Һ��Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ�� ��
��3��A�ж���ͬ���칹�壬ͬʱ��������Ҫ���A��ͬ���칹�干�� �֡�
��1mol��ͬ���칹������5molNaOH������Ӧ
�ں˴Ź���������ʾ���ĸ����շ�
��4����ͪ����һ�ֳ����л��ϳ��м��塣��ʯ���ѽ����ɷ�֮һΪԭ�ϣ�ͨ�����������Ƶñ�ͪ�ᣬ�����ϳɸ߾���K��

��д���������ʵĽṹ��ʽ��D�� M�� ��
���ڢ١������У����ڼӳɷ�Ӧ���� ���ñ����д��
�۵ڢڲ��Ļ�ѧ��Ӧ����ʽΪ ��
��1�����ӣ��ǻ� ���� ����1�֣����ӡ���д���۷֣����ִ�����ýṹ��ʽ��ʾ���÷֣�
��2��
 ��5NaOH��
��5NaOH�� ��
�� ��3H2O ��3�֣�����ƽ��1�֣�
��3H2O ��3�֣�����ƽ��1�֣� ��3��2�� ��2�֣�
��4�� �� D��CH3CH��CH2 M��CH2��CHCOOCH3 ����2�֣�
�ڢ٢� ��2�֣�©ѡ1����1�֣���ѡ���÷֣�
�� CH3CHBrCH2Br��2NaOH
 CH3CHOHCH2OH��2NaBr ��2�֣�
CH3CHOHCH2OH��2NaBr ��2�֣�����������ɷ���ģ�ͣ��ٸ��ݷ���ʽC10H6O6�����Եó������ʵĽṹʽΪ��
 �����л���������������ˮ������A��
�����л���������������ˮ������A�� �ͱ�ͪ�
�ͱ�ͪ� ��
����1���ɸ��л���Ľṹ֪�京��������Ϊ�����ǻ���������
��2�������������Ʒ�Ӧ�Ĺ������з��ǻ������������Է�Ӧ�Ļ�ѧ����ʽΪ��
 ��5NaOH��
��5NaOH�� ��
�� ��3H2O
��3H2O ��3��A�ķ���ʽΪ��C7H6O5�������Ͷ�Ϊ5���������Ƶ�����A��ͬ���칹����һ�����֣�


��4����ת��ͼ֪��Dͨ������ת���õ���ͪ�ᣬ�����ݷ�Ӧ��������D��ֻ������̼ԭ�ӣ�������һ��̼̼˫��������D�ĽṹΪ��CH2=CH-CH3��E�ĽṹΪ��
 ��F�ĽṹΪ��
��F�ĽṹΪ�� ��C�ĽṹΪ��
��C�ĽṹΪ�� ��H�ĽṹΪ��
��H�ĽṹΪ�� ��M�ĽṹΪ��
��M�ĽṹΪ�� ��K��M�ļӾ۷�Ӧ���
��K��M�ļӾ۷�Ӧ����� D��M�Ľṹ���ϡ�
�ڷ����ӳɷ�Ӧ���Ǣ٢� ��
�۵ڢڲ�����±�����ڼ��������µ�ˮ�ⷴӦ������ʽΪ��
CH3CHBrCH2Br��2NaOH
 CH3CHOHCH2OH��2NaBr
CH3CHOHCH2OH��2NaBr 
��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ
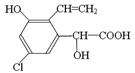 �����й��ڸ��л����˵���в���ȷ����
�����й��ڸ��л����˵���в���ȷ����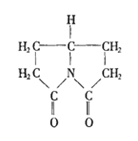
 ������������11��̼ԭ�Ӵ���ͬһƽ��
������������11��̼ԭ�Ӵ���ͬһƽ�� ��1 mol H2�����ӳɷ�Ӧ�ɵõ�3�ֲ�ͬ����
��1 mol H2�����ӳɷ�Ӧ�ɵõ�3�ֲ�ͬ����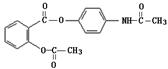 ����������ʹ����Ҫ�������ʪ�Թؽ��ס���ʪʹ����ð���յȵ����ơ���ŵ�����ɰ�˾ƥ�֣�I��������Ϣʹ��II�����������ӣ��ϳɡ�
����������ʹ����Ҫ�������ʪ�Թؽ��ס���ʪʹ����ð���յȵ����ơ���ŵ�����ɰ�˾ƥ�֣�I��������Ϣʹ��II�����������ӣ��ϳɡ�
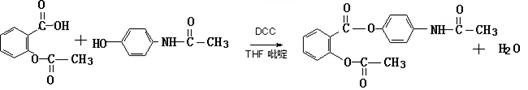
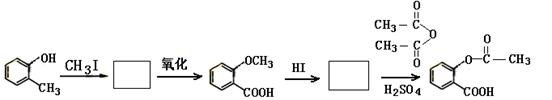
 �ڴ�������Ҳ��������Ϣʹ��II���������Ƣٵķ�Ӧ������Ľṹ��ʽΪ��_________________________��
�ڴ�������Ҳ��������Ϣʹ��II���������Ƣٵķ�Ӧ������Ľṹ��ʽΪ��_________________________�� �� �����й�˵����ȷ���ǣ� ��
�� �����й�˵����ȷ���ǣ� ��