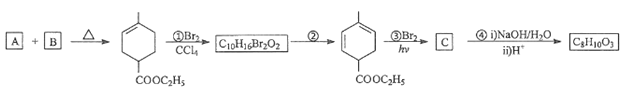
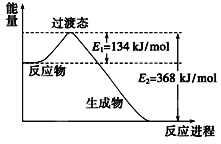
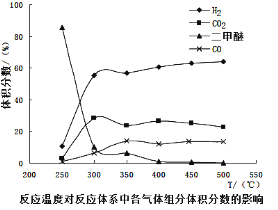
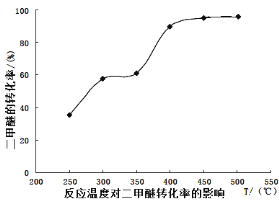
��Ŀ����
����Ŀ��20��ʱ��10mL0.1mol��L-1������Һ�в��ϵ���0.1mol��L-1NaOH��Һ����ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ�������
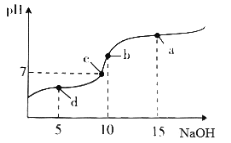
A. a�㣺c(Na+)>c(CH3COO-)>c(OH-)>c(H+)
B. b�㣺 c(H+)= c(CH3COOH)+c(OH-)
C. c�㣺c(Na+)= c(CH3COO-)>c(H+)=c(OH-)
D. d�㣺c(CH3COO-)>c(Na+)>c(H+)>c(OH-)
���𰸡�B
��������A��a��ΪNaOH��CH3COONa�Ļ�����2c��NaOH��=c��CH3COONa������Һ�ʼ��ԣ����������ˮ�ˮ��̶Ƚ�С��������Һ������Ũ�ȴ�С˳����c��Na+����c��CH3COO-����c��OH-����c��H+������A��ȷ��B��b��ʱ����Һ�ʼ��ԣ�Ӧ��c��H+����c��OH-������B����C��������Һ����غ��֪��Һ��Ӧ����c��Na+��+c��H+��=c��CH3COO-��+c��OH-������Һ�����ԣ�Ӧ��c��H+��=c��OH-������c��Na+��=c��CH3COO-����������Һ������Ũ�ȹ�ϵ��c��Na+��=c��CH3COO-����c��H+��=c��OH-������C��ȷ��D��d��ʱ�����������ҺΪCH3COOH��CH3COONa�Ļ�����Һ�����ԣ�����c��H+����c��OH-������Һ�ʵ����ԣ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��Na+����c��CH3COO-������c��CH3COO-����c��Na+����c��H+����c��OH-������D��ȷ����ѡB��