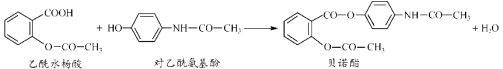
��Ŀ����
����Ŀ����Zn����Cu���õ������Ӻ���ij�������Һ�У���������ͼ��ʾװ�á��Իش��������⣺
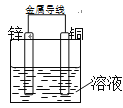
��1�����������ҺΪϡ���ᣬ��Zn��Ϊԭ��ص�______���������������������_______��Ӧ�����������ԭ������ �缫��ӦʽΪ___________________��Cu���Ͽɹ۲쵽��������______________________���缫��ӦʽΪ______________________________��
��2���������Ϊ����ͭ��Һ����Cu��Ϊԭ��ص�______���������������������_________��Ӧ�����������ԭ��������缫��ӦʽΪ____________________________________��
��3�������������������У�Zn�����ٵ�������ȣ���Cu���ϣ�1���ͣ�2�����������ʵ�����֮��Ϊ_____________��
���𰸡� �� ���� Zn - 2e- == Zn2+ �д������ݲ��� 2H+ + 2e- == H2�� �� ��ԭ Cu2+ + 2e- == Cu 1:32
����������1����Zn����Cu���õ������Ӻ����������ҺΪϡ���ᣬ��пΪ����������ʧȥ���ӵ�������Ӧ��������ӦΪ��Zn-2e-=Zn2+��ͭ������������Һ�е������ӷŵ�����������������д������ݲ�����������ӦʽΪ2H+ + 2e-��H2������2����Zn����Cu���õ������Ӻ��������Ϊ����ͭ��Һ�������ͭ���������ü�������ͭ����ӦΪ��Cu2++2e-=Cu��������ԭ��Ӧ����3�����������������ж�����1molZn����Cu���ϣ�1�����������ʵ�����Ϊ��1mol��2g/mol=2g����2�����������ʵ�����Ϊ��1mol��64g/mol=64g��Cu���ϣ�1���ͣ�2�����������ʵ�����֮��Ϊ2g��64g=1��32���ʴ�Ϊ��1��32��