��Ŀ����
7����֪����ͬ������Ԫ����ɵ�������A��B��C��D���������������࣬A��B��C��D�ĵ����������A��B��C��D��������ܣ�����D�еĵ�����������������D1����ҽ������Һ�� ����B1�ķе��B2�ߣ�| �� | A1 | B1 | C1 | D1 |
| ������ | 10 | 10 | 10 | 18 |
| �� | A2 | B2 | C2 | D2 |
| ������ | 10 | 10 | 10 | 18 |
��1��������������������Ԫ���У�ԭ�����������м��Ԫ����Ԫ�����ڱ���λ���ǵڶ����ڵ�VA�壻 D1�ĵ���ʽΪ��
 ��
����2��Һ̬��B2��Na��Ӧ�ķ���ʽ��2Na+2NH3��l��=2NaNH2+H2����
��3�����������ƣ�NaClO2����Ҫ�������ġ���ֽҵ��Ư����Ҳ����ʳƷ������ˮ�����ȣ��Ʊ��������ƣ����Խ�ClO2����ͨ��D1��NaOH�Ļ��Һ�У���д���Ʊ�����ʽ2ClO2+2NaOH+H2O2=2NaClO2+2H2O+O2������D1�������ǻ�ԭ����
�����â���ԭ���Ʊ���NaClO2•3H2O����������������á���ӵ��������ⶨ��������������I-������Ӧ�����������ʣ��Ĵ��ȣ��������£���֪��I2+2S2O32-=S4O62-+2I-����
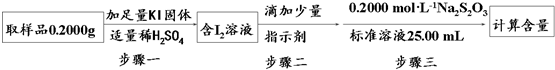
����һ�����ӷ���ʽΪClO2-+4I-+4H+=2I2+Cl-+2H2O���������ָʾ���ǵ��ۣ��������г�����Һ����ɫ����ɫ���Ұ���Ӳ���ɫ��������ʱ���ﵽ�ζ��յ㣻�����������NaClO2•3H2O�������ٷ���Ϊ90.3%��
���� ��ͬ������Ԫ����ɵ�������A��B��C��D���������������࣬A��B��C����10���ӣ�DΪ18���ӣ�Ӧ��H��O��H��N�γɵ�����H��O�γɵ���A��B��C��DΪ�ֱ�Ϊ��OH-��H2O��H3O+��H2O2��H��N�γɵ���A��B��C��DΪ�ֱ�Ϊ��NH2-��NH3��NH4+��N2H4��B1�ķе��B2�ߣ���B1ΪH2O��B2ΪNH3���ʣ�
H��O�γɵ���Ϊ��һ�飬��A1ΪOH-��B1ΪH2O��C1ΪH3O+��D1ΪH2O2��
H��N�γɵ���Ϊ�ڶ��飬��A1ΪNH2-��B2ΪNH3��C2ΪNH4+��D2ΪN2H4��
��1��ԭ�����������м�ΪNԪ�أ�Nԭ����2�����Ӳ㣬����������Ϊ5���ݴ�ȷ����Ԫ�����ڱ��е����ʣ�
D2ΪN2H4��������Nԭ��֮���γ�1�Թ��õ��Ӷԣ�Hԭ����Nԭ��֮���γ�1�Թ��õ��Ӷԣ�
��2��Na��NH3��l����Ӧ����NaNH2��H2��
��3��������Ŀ��֪��ClO2����ͨ��H2O2��NaOH�Ļ��Һ������NaClO2��ClԪ�ػ��ϼ۽��ͣ�����ԭ����H2O2����������Ӧ����O2��ͬʱ��ˮ���ɣ�
��������ͼ��֪��ClO2-����������������I-����I2���������۱���ɫ�����������ָʾ�������еζ�������Һ����ɫ����ɫ���Ұ���Ӳ���ɫ����ʱ���ﵽ�ζ��յ㣻
����Ʒ�Ĵ���Ϊa����NaClO2•3H2O����������Ϊ0.2ag�����ݹ�ϵʽ�з��̼��㣮
��� �⣺��ͬ������Ԫ����ɵ�������A��B��C��D���������������࣬A��B��C����10���ӣ�DΪ18���ӣ�Ӧ��H��O��H��N�γɵ�����H��O�γɵ���A��B��C��DΪ�ֱ�Ϊ��OH-��H2O��H3O+��H2O2��H��N�γɵ���A��B��C��DΪ�ֱ�Ϊ��NH2-��NH3��NH4+��N2H4��B1�ķе��B2�ߣ���B1ΪH2O��B2ΪNH3���ʣ�
H��O�γɵ���Ϊ��һ�飬��A1ΪOH-��B1ΪH2O��C1ΪH3O+��D1ΪH2O2��
H��N�γɵ���Ϊ�ڶ��飬��A1ΪNH2-��B2ΪNH3��C2ΪNH4+��D2ΪN2H4��
��1��ԭ�����������м�ΪNԪ�أ�Nԭ����2�����Ӳ㣬����������Ϊ5���������ڱ��еڶ����ڵ�VA�壬D1ΪH2O2�������ʽΪ�� ��
��
�ʴ�Ϊ���ڶ����ڵ�VA�壻 ��
��
��2��Na��NH3��l����Ӧ����NaNH2��H2����Ӧ����ʽΪ��2Na+2NH3��l��=2NaNH2+H2����
�ʴ�Ϊ��2Na+2NH3��l��=2NaNH2+H2����
��3��������Ŀ��֪��ClO2����ͨ��H2O2��NaOH�Ļ��Һ������NaClO2��ClԪ�ػ��ϼ۽��ͣ�����ԭ����H2O2����������Ӧ����O2��ͬʱ��ˮ���ɣ���Ӧ����ʽΪ��2ClO2+2NaOH+H2O2=2NaClO2+2H2O+O2����Ӧ��H2O2�ǻ�ԭ����
�ʴ�Ϊ��2ClO2+2NaOH+H2O2=2NaClO2+2H2O+O2����ԭ����
��������ͼ��֪��ClO2-����������������I-����I2��ClO2-����ԭΪCl-��ͬʱ����H2O����Ӧ���ӷ���ʽΪ��ClO2-+4I-+4H+=2I2+Cl-+2H2O��
�������۱���ɫ�����������ָʾ����
���еζ�������Һ����ɫ����ɫ���Ұ���Ӳ���ɫ����ʱ���ﵽ�ζ��յ㣬
����Ʒ�Ĵ���Ϊa����NaClO2•3H2O����������Ϊ0.2ag����
NaClO2•3H2O��2I2��4S2O32��
144.5g 4mol
0.2ag 0.2mol/L��0.025L
����144.5g��0.2ag=4mol��0.2mol/L��0.025L
���a=90.3%��
�ʴ�Ϊ��ClO2-+4I-+4H+=2I2+Cl-+2H2O�����ۣ���Һ����ɫ����ɫ���Ұ���Ӳ���ɫ����90.3%��
���� ���⿼�������ƶϡ���ѧ���������ԭ��Ӧ�ζ�����ѧ����ȣ��ѶȽϴ��ƶ������ǽ���Ĺؼ���ע�����ճ���10���ӡ�18��������

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�| A�� | V��=102V�� | B�� | V��=102 V�� | C�� | V��=2 V�� | D�� | V��=2V�� |
| A�� | �۵㣺CO2��H2O��SiO2��KCl | B�� | �ȶ��ԣ�H2O��NH3��PH3��SiH4 | ||
| C�� | ���ԣ�H3PO4��H2SO4��HClO4��H2SiO3 | D�� | ���Ӱ뾶��K+��Na+��Mg2+��Al3+ |
| A�� | ��������Һ��ͭ��Ӧ��Cu+Ag+�TCu2++Ag | |
| B�� | ����þ�����ᷴӦ��O2-+2H+�TH2O | |
| C�� | ̼��������ᷴӦ��CO${\;}_{3}^{2-}$+2H+�TH2O+CO2�� | |
| D�� | Fe��OH��3����H2SO4��Һ�У�3H++Fe��OH��3�TFe3++3H2O |
| A�� | ����ˮ���γɵ�Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| B�� | �ں������������п�飬�ɼ�������ĸ�ʴ���ʣ������������������������� | |
| C�� | ���ݷ�ɢ�����ӵĴ�С���࣬����ɢϵ��Ϊ��Һ����Һ������ | |
| D�� | Ϊ��ֹ�±��ȸ�֬��ʳƷ�����������ڰ�װ�з��뻹ԭ���۵� |
| A�� | ̼��������ᷴӦ��CO32-+2H+�TCO2��+H2O | |
| B�� | ����ϡ���ᷴӦ��Fe+2H+�TFe2++H2�� | |
| C�� | ͭƬ������������Һ�� Cu+Ag+�TCu2++Ag | |
| D�� | ����������Һ�еμ�ϡ���OH-+H+�TH2O |
| A�� | �������������ᷴӦ��H++OH-�TH2O | |
| B�� | ��С�մ�����θ����ࣺHCO3-+H+�TCO2��+H2O | |
| C�� | �������ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| D�� | CaCO3����ϡ�����У�CO32-+2H+�TCO2��+H2O |
 ij��̬��A�ڱ�״���µ��ܶ���1.875g/L����˴Ź�������������壬�������Ϊ2��1��3��A����ͼת����ϵ��
ij��̬��A�ڱ�״���µ��ܶ���1.875g/L����˴Ź�������������壬�������Ϊ2��1��3��A����ͼת����ϵ��