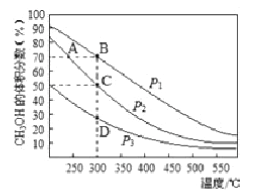
��Ŀ����
����Ŀ��ij�о���ѧϰС�����ⶨ������(25����101 kPa)������Ħ���������ش��������⡣��С����Ƶļ���ʵ��װ����ͼ��ʾ��
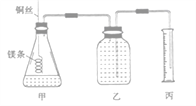
��ʵ�����Ҫ�����������£�
(1)����80 mL 1.0 mol��L-1��������Һ��
��ͨ�����㣬������Ͳ��ȡ18mol/L��Ũ��������Ϊ__________mL��
��������ϡ����Ĺ����У�����Ҫ��ʵ�������У��ձ�����Ͳ����������__________��________��
�����в��������������Һ�����ʵ���Ũ��ƫ�����________��
A.ת����Һʱ����������Һ����������ƿ����
B.ת��ǰ������ƿ������������ˮ
C.����ʱ���۾����ӿ̶���
D.���ݺ�����ҡ�Ⱥ�Һ���½�����������ˮ
(2)����Ͳ��ȡ25.0mL 1.0 mol��L-1��������Һ������ƿ�У�����ȡa g����ȥ��������Ĥ��þ������ϵ��ͭ˿ĩ�ˣ�ΪʹH2SO4ȫ���μӷ�Ӧ��a����ֵ����Ϊ__________��
(3)�����ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ���Ӧ���������ϵ�¶Ȼָ������£�������Ͳ��ˮ�����ΪVmL������ʱ���ָ��������⣬��Ҫע�⣺___________________���Ҹò���Ӧѡ��______________(�����)����Ͳ��
A.100mL B.200 mL C.500 mL D.1000mL
(4)������ˮ������Ӱ�죬��ʵ�������²������Ħ������ļ���ʽΪVm=____________(��V��ʾ)����δ��ȥþ�����������Ĥ����������____________��(����ƫ�������� ƫС��������Ӱ����)��
���𰸡� 5.6 ��ͷ�ι� l00mL����ƿ C 0.6 �ҡ���װ����Һ����ƽ D Vm=V/25 L/mol ƫС
����������1����û��80mL����ƿ����Ҫ����100mL��Һ����������Ͳ��ȡ18mol/L��Ũ��������Ϊ![]() mL����������Һ��Ҫ�IJ����Ǽ��㡢��ȡ���ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ҡ�������ݵȲ��裬�������Ҫ��ʵ�������У��ձ�����Ͳ����������l00mL����ƿ�ͽ�ͷ�ιܣ���A.ת����Һʱ����������Һ����������ƿ���������ʼ��٣�Ũ��ƫ�ͣ�A������B.ת��ǰ������ƿ������������ˮ����Ӱ����B������C.����ʱ���۾����ӿ̶�����Һ���ڿ̶����·�����Һ������٣�Ũ��ƫ�ߣ�C��ȷ��D.���ݺ�����ҡ�Ⱥ�Һ���½�����������ˮ����Һ������ӣ�Ũ��ƫ����D����ѡC����2����������ʵ�����0.025mol�����ݷ���ʽMg+H2SO4=MgSO4+H2����֪��Ҫ����þ�����ʵ�����0.025mol��������0.025mol��24g/mol��0.6g����3����������������ѹǿӰ�����˶���ʱ���ָ��������⣬��Ҫע���ҡ���װ����Һ����ƽ�����ɵ�������0.025mol����״���µ������0.025mol��22.4L/mol��0.56L������Ӧѡ��1000mL��Ͳ����ѡD����4����Ӧ�ܹ�����0.025mol�����������������ΪVmL��������ˮ������Ӱ�죬���ڸ�ʵ�������²������Ħ������ļ���ʽΪ��Vm=V��n=0.001VL��0.025mol��V/25 Lmol-1����δ��ȥþ�����������Ĥ���������ɵ�����������٣���������ƫС��
mL����������Һ��Ҫ�IJ����Ǽ��㡢��ȡ���ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ҡ�������ݵȲ��裬�������Ҫ��ʵ�������У��ձ�����Ͳ����������l00mL����ƿ�ͽ�ͷ�ιܣ���A.ת����Һʱ����������Һ����������ƿ���������ʼ��٣�Ũ��ƫ�ͣ�A������B.ת��ǰ������ƿ������������ˮ����Ӱ����B������C.����ʱ���۾����ӿ̶�����Һ���ڿ̶����·�����Һ������٣�Ũ��ƫ�ߣ�C��ȷ��D.���ݺ�����ҡ�Ⱥ�Һ���½�����������ˮ����Һ������ӣ�Ũ��ƫ����D����ѡC����2����������ʵ�����0.025mol�����ݷ���ʽMg+H2SO4=MgSO4+H2����֪��Ҫ����þ�����ʵ�����0.025mol��������0.025mol��24g/mol��0.6g����3����������������ѹǿӰ�����˶���ʱ���ָ��������⣬��Ҫע���ҡ���װ����Һ����ƽ�����ɵ�������0.025mol����״���µ������0.025mol��22.4L/mol��0.56L������Ӧѡ��1000mL��Ͳ����ѡD����4����Ӧ�ܹ�����0.025mol�����������������ΪVmL��������ˮ������Ӱ�죬���ڸ�ʵ�������²������Ħ������ļ���ʽΪ��Vm=V��n=0.001VL��0.025mol��V/25 Lmol-1����δ��ȥþ�����������Ĥ���������ɵ�����������٣���������ƫС��