��Ŀ����
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㣮��1��ʵ���ã�1g�״��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��
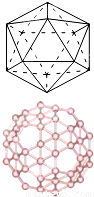
��2���Է��ָ���ϩC60������������Ľṹ���������������Ƕ�̼ԭ���Ŵع㷺��������о���C60������������״�Ķ����壬�ýṹ�Ľ����������¿��ǣ���ȥ��ͼ�����壨��12�����㣬20���������Σ���ÿһ�����ǣ��õ���һ����Ŀ��������κ�������������ɵĶ����壬��ΪC60���ӣ��ö������к���90���⣬��ÿ��̼ԭ�Ӷ��ﵽ��8���ӵ��ȶ��ṹ����֪���ֹ��ۼ��ļ����������±���
�����±����ݹ����C60���ӵ�ȼ����Ϊ ��
| ��ѧ�� | C-C | C=C | C��C | C=O | O=O |
| ����/ | a | b | c | d | e |
���𰸡���������1�����ݻ�ѧ����ʽ����ƽϵ�������Ӧ���µķ�Ӧ�ȣ�ע���ע���ʾۼ�״̬��
��2�����ȿɴ�2��������ж�C60���Ӻ���̼̼˫����һ�Ǵ������Ϸ�����������ϩ���еġ�ϩ����˵�������к���̼̼˫�������Ǵӽṹ�Ϸ���������C60������ÿ��̼ԭ�Ӷ��ﵽ��8���ӵ��ȶ��ṹ�����ÿ��̼ԭ�ӵ����ӷ�ʽ��Ϊ���ʷ����к���̼̼˫����ͬ��������C60��������̼̼������
��3��������ֻҪ���C60�����к��е���������Ŀ���ɵõ��𰸣��ɣ�1������Ϣ��֪������ȥһ�����Ǻõ�һ��������Σ�����12�����㣬�ʵõ����������Ϊ12��ԭ��������漴Ϊ��ȥ���Ǻ����õ���������������������������ĿΪ20������n����20+60=80��
����⣺��1��1g�״�ȼ������CO2��Һ̬ˮʱ����22.7kJ������64g�״�ȼ������CO2��Һ̬ˮʱ����1452.8kJ�������Ȼ�ѧ����ʽ����д����д����ע���ע���ʵľۼ�״̬�ͷ�Ӧ�ķ�Ӧ�ȣ����Է�Ӧ���Ȼ�ѧ����ʽΪ��2CH3OH��l��+3O2��g�� 2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1��
2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1��
�ʴ�Ϊ��2CH3OH��l��+3O2��g�� 2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1 ��
2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1 ��
��2�����ȿɴ�2��������ж�C60���Ӻ���̼̼˫����һ�Ǵ������Ϸ�����������ϩ���еġ�ϩ����˵�������к���̼̼˫�������Ǵӽṹ�Ϸ���������C60������ÿ��̼ԭ�Ӷ��ﵽ��8���ӵ��ȶ��ṹ�����ÿ��̼ԭ�ӵ����ӷ�ʽ��Ϊ���ʷ����к���̼̼˫����ͬ��������C60��������̼̼��������ʾC60���ӵ�ȼ���ȵ��Ȼ�ѧ����ʽΪC60��s��+60O2��g��=60CO2��g����������ü���������÷�Ӧ�ķ�Ӧ��ʱ�����������C60�����к��е�̼̼˫����̼̼����������C60���ӵĽṹ�ص��֪��n��������+n��˫����=90���������������̼ԭ�ӵ����ӷ�ʽ�ɵ�n��˫����=60/2=30����n��������=60���÷�Ӧ�ġ�H=��Ӧ��ļ���֮��-������ļ���֮��=-��120d-60a-30b-60e�� kJ?mol-1������ȼ����Ϊ��120d-60a-30b-60e�� kJ?mol-1���ʴ�Ϊ����120d-60a-30b-60e�� kJ?mol-1��
��3��������ֻҪ���C60�����к��е���������Ŀ���ɵõ��𰸣��ɣ�1������Ϣ��֪������ȥһ�����Ǻõ�һ��������Σ�����12�����㣬�ʵõ����������Ϊ12��ԭ��������漴Ϊ��ȥ���Ǻ����õ���������������������������ĿΪ20������n����20+60=80��
�ʴ�Ϊ��80��
���������⿼�����Ȼ�ѧ����ʽ����д����Ӧ�ã�����ṹ�ķ����жϺͼ���Ӧ�ã�ȼ���ȵĸ��������ȼ���Ⱥͻ�ѧ�����ܵļ����ϵ���ؼ��Ǹ���ϩ�Ľṹ�����������Ϣ��Ӧ�ã���Ŀ�Ѷ��еȣ�
��2�����ȿɴ�2��������ж�C60���Ӻ���̼̼˫����һ�Ǵ������Ϸ�����������ϩ���еġ�ϩ����˵�������к���̼̼˫�������Ǵӽṹ�Ϸ���������C60������ÿ��̼ԭ�Ӷ��ﵽ��8���ӵ��ȶ��ṹ�����ÿ��̼ԭ�ӵ����ӷ�ʽ��Ϊ���ʷ����к���̼̼˫����ͬ��������C60��������̼̼������
��3��������ֻҪ���C60�����к��е���������Ŀ���ɵõ��𰸣��ɣ�1������Ϣ��֪������ȥһ�����Ǻõ�һ��������Σ�����12�����㣬�ʵõ����������Ϊ12��ԭ��������漴Ϊ��ȥ���Ǻ����õ���������������������������ĿΪ20������n����20+60=80��
����⣺��1��1g�״�ȼ������CO2��Һ̬ˮʱ����22.7kJ������64g�״�ȼ������CO2��Һ̬ˮʱ����1452.8kJ�������Ȼ�ѧ����ʽ����д����д����ע���ע���ʵľۼ�״̬�ͷ�Ӧ�ķ�Ӧ�ȣ����Է�Ӧ���Ȼ�ѧ����ʽΪ��2CH3OH��l��+3O2��g��
 2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1��
2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1���ʴ�Ϊ��2CH3OH��l��+3O2��g��
 2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1 ��
2CO2��g��+4H2O��l��?H=-1452.8 kJ?mol-1 ����2�����ȿɴ�2��������ж�C60���Ӻ���̼̼˫����һ�Ǵ������Ϸ�����������ϩ���еġ�ϩ����˵�������к���̼̼˫�������Ǵӽṹ�Ϸ���������C60������ÿ��̼ԭ�Ӷ��ﵽ��8���ӵ��ȶ��ṹ�����ÿ��̼ԭ�ӵ����ӷ�ʽ��Ϊ���ʷ����к���̼̼˫����ͬ��������C60��������̼̼��������ʾC60���ӵ�ȼ���ȵ��Ȼ�ѧ����ʽΪC60��s��+60O2��g��=60CO2��g����������ü���������÷�Ӧ�ķ�Ӧ��ʱ�����������C60�����к��е�̼̼˫����̼̼����������C60���ӵĽṹ�ص��֪��n��������+n��˫����=90���������������̼ԭ�ӵ����ӷ�ʽ�ɵ�n��˫����=60/2=30����n��������=60���÷�Ӧ�ġ�H=��Ӧ��ļ���֮��-������ļ���֮��=-��120d-60a-30b-60e�� kJ?mol-1������ȼ����Ϊ��120d-60a-30b-60e�� kJ?mol-1���ʴ�Ϊ����120d-60a-30b-60e�� kJ?mol-1��
��3��������ֻҪ���C60�����к��е���������Ŀ���ɵõ��𰸣��ɣ�1������Ϣ��֪������ȥһ�����Ǻõ�һ��������Σ�����12�����㣬�ʵõ����������Ϊ12��ԭ��������漴Ϊ��ȥ���Ǻ����õ���������������������������ĿΪ20������n����20+60=80��
�ʴ�Ϊ��80��
���������⿼�����Ȼ�ѧ����ʽ����д����Ӧ�ã�����ṹ�ķ����жϺͼ���Ӧ�ã�ȼ���ȵĸ��������ȼ���Ⱥͻ�ѧ�����ܵļ����ϵ���ؼ��Ǹ���ϩ�Ľṹ�����������Ϣ��Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ