��Ŀ����
��9�֣���ȩ(CH3CHO)�ڴ������ڵ������£����Ա��������������ᡣ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ����ͼ��ʾ���Թ�A��װ��40%����ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ�壩����֪��60��~80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���Σ���Ӧ������ȫ���й����ʵķе���£�
| ���� | ��ȩ | ���� | ���� | �Ҵ� | ˮ |
| �е�/�� | 20.8 | 117.9 | 290 | 78.2 | 100 |
��1���Թ�A����60��~80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������
������_________________________________________��
��2����ͼ��ʾ����ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ��
�ȼ�ˮ�����λ��Ӧ��________�����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ��_______��
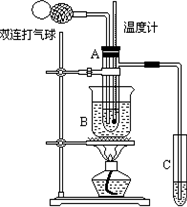
��3���ձ�B��ʢװ��Һ�������_______��
��4���Թ�C���ռ������Ǵֲ�Ʒ�������һ���ᴿ���ɲ��õķ����ǣߣߣߣߣߣ�
�������ᴿ��IJ�Ʒ�����������н��вⶨ�����ʺɱ�����ǣߣߣߣߣ�����ֵ����
���������ں˴Ź������н��вⶨ����˴Ź��������Уߣ��ַ壬�����֮��Ϊ�ߣߡ�
��1��2CH3CHO+O2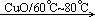 2CH3COOH��
2CH3COOH��
��2�������Թ�A�ķ�ӦҺ�С��Թ�A��֧�ܿڴ���
��3�����͡�����4������60��2��3��1��1��3��
����

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
��֪��(1)���������ڴ������������¿ɷ����������Ҵ�������������
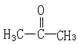 |
|||
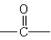 |
|||
(2)�ʻ�( ) �����������������ɵĻ������ͪ(���ͪ )�����ֱ�
�����������������ɵĻ������ȩ������ȩCH3CHO�������д�������ʱ�������ܵõ�ȩ�������� �� ��
A��(CH3)2CHOH B��(CH3)2CHCH2OH
C��CH3CH2CH2OH D��(CH3)3CCH2OH
