��Ŀ����
����Ŀ��N2(g)��H2(g)�����������澭�����¹�������NH3(g)��
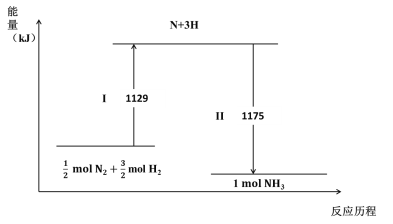
����˵������ȷ����
A�����������ƻ��ľ�Ϊ�Ǽ��Լ�
B������������������II���̷ų�����
C�� N2(g) + 3H2(g) ![]() 2NH3(g) ��H = �C44 kJ��mol-1
2NH3(g) ��H = �C44 kJ��mol-1
D��1mol N2(g)��3 mol H2(g)�������������2 mol NH3(g) �������������
���𰸡�C
��������
���������A�������������ж��к��зǼ��Լ�������������У��ƻ��ľ�Ϊ�Ǽ��Լ�����A��ȷ��B���Ͽ���ѧ����Ҫ�����������γɻ�ѧ����Ҫ�ų���������B��ȷ��C�� N2(g) + 3H2(g) ![]() 2NH3(g) ��H = 2��(1129-1175)=�C88 kJ��mol-1����C����D������ͼ�÷�ӦΪ���ȷ�Ӧ�����1mol N2(g)��3 mol H2(g)�����е���������2 mol NH3(g) �����е��������ߣ���D��ȷ����ѡC��
2NH3(g) ��H = 2��(1129-1175)=�C88 kJ��mol-1����C����D������ͼ�÷�ӦΪ���ȷ�Ӧ�����1mol N2(g)��3 mol H2(g)�����е���������2 mol NH3(g) �����е��������ߣ���D��ȷ����ѡC��

��ϰ��ϵ�д�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
�����Ŀ